| 0) Reserve 1
week on the CCD/RaxisIV++
1) Bring in VCR/TV for cryo video. Set up in Kim's office.
Print out the handout datacoparam_small.jpg
Print out the demo version datacoparam.ppt
2) Setup microscope near F-RD/CCD, with following supplies:
- liquid nitrogen filling dewar
- short dewar
- tall dewar
- cryo tongs
- two forceps
- glass depression dish
- P-20 pipetman
- glycerol
- tips
- gloves
- cryopins
- razor blade
- crystal trays
- ruler
- cryovial
- cryocane
3) Log into fermat and have XRayView running.
4) Lay out rotating anode and filament for demonstration
5) Reserve Harker for XRayView Demo.
Course of events:
- show video
- talk about lab x-ray safety
- demonstrate how x-rays are produced with filament and copper
anode.
- demonstrate cryo screening (clear vs. opaque)
- demonstrate mounting and freezing a crystal
- Have kids do cryo cooling, check their work with diffraction
shot.
- Mount a crystal for data collection on CCD and imaging
plate.
- discuss data collection parameters (1)distance (2)oscillation
angle (3)exposure time(4) number of frames collected
- demo with XRayView
Outline:
Today we are going to (1) see a short video about cryo-crystallographic
techniques. These are techniques that allow us to cool crystals to
-180 degrees C for data collection. Cryo-cooling is important because
it slows down the x-ray induced decay in the crystal, preserving the crystal
for hours of data collection. (2) Duilio will show us how x-rays are produced
in our laboratory and describe the equipment. (3) You'll test your crystallization
conditions to see whether the addition of a cryoprotectant (anti-freeze)
is necessary. (4) You'll cryo-protect your crystals. (5) Cryo-cool
them in a nitrogen stream. (6) Begin a short data collection. (7) Learn how
to remove the crystal for storage or shipping to a synchrotron source. (8)
Restore the crystal to the x-ray camera after shipping.
Show video
Mike (How to scoop up a crystal) Using paper mache crystal
and mega loop made from a paper clip and drinking straw.
(1) If the crystal is floating on the surface of the drop, it is easy to
just put the loop under the crystal and lift it out.
(2) If the crystal is on the bottom (most big crystals are) then avoid the
tendency to push the crystal to the side of the drop. Youll just push
the crystal off the coverslip. Instead, keep the crystal in the center
of the drop. Use the loop like a broom to sweep the crystal up off the bottom
of the coverslip. Use a wafting motion. The crystal will be momentarily
floating, but will eventually sink to the bottom again. While the crystal
is floating, stick the loop under the crystal and lift the crystal out of
the loop.
(3) If the crystal is smaller than the loop -- again you need to get the
crystal off the bottom. Once it is off the bottom, turn the loop
on the side, so the crystal tumbles over the loop and gets trapped
inside. The bottom edge of the loop will support the crystal's weight.
Duilio (How x-rays are produced in the lab):
(1) Tungsten filament is heated by a current to produce free electrons.
(2) Electron are accelerated to copper surface, ejecting K shell electrons
from copper nuclei.
(3) Ejected electrons fall back into orbit, giving off photons (x-rays)
at 1.5418 Angstrom wavelength.
(4) All done in vacuum inside the tower.
(5) Rotation and cooling water help cool the anode, prevent meltdown. The
tower contains the x-rays. Shutter prevents the x-rays from escaping.
(6) Xrays exit the tower when the shutter is open. Light indicates this.
(7)X-rays are collimated in the collimator, so the photons are directed to
the crystal.
(8) The crystal sits in a loop on a pin on a goniometer head on a goniometer.
(9) 5% of the x-rays are scattered by the crystal. The beam stop will absorb
the remaining 95% of the x-rays which are not diffracted by the crystal.
Protects the detector from saturation.
(10) The detector captures the diffracted x-rays and records them.
(11) The cryo stream cools the crystal.
(12) When mounting a cryopin on the goniometer head, take care not to bump
the collimator, cryo-stream, or beam stop.
Mike (How to test for the need to add cryo-protectant)
(1)Find the crystal you want to use for diffraction.
(2)Carefully remove the glass coverslip and set it down on the plastic lid.
Dip the loop in the reservoir to coat it with a film. Replace
the coverslip on the reservoir and put the loop in the cryo-stream. Observe
whether opaque or clear.
(3) repeat with 65% reservoir, 35% glycerol. Because ice is bad.
(4) Transfer crystal to 10 uL cryoprotected reservoir solution.
(5) Transfer crystal to goniometer head.
(6) Center crystal with 3-translations.
Duilio (How to transfer crystals for shipping)
(1) Once you have screened your crystal for diffraction quality and decide
that it is worthy of making the trip to the sychrotron for data collection,
you must transfer the crystal 600 miles away with out changing its temperature.
Cryo-tools allow you to do this.
(2) Temperature changes could potentially destroy the crystal by making the
lattice expand and contract. Ice could form too. Ice has its
own diffraction pattern that will overlap with the protein diffraction pattern.
(3) Cryo-tongs insulate crystal from heat while you transfer from goniometer
head to liquid nitrogen dewar.
(4) Cryo-vial provides a liquid nitrogen bath for the crystal while the
crystal is being transferred onto a storage cane (cryo-cane). Use magnetic
want and clamps for manipulation of the pin and vial.
(5) Hole through base of pin allows gaseous nitrogen to escape.
(6) Vials must be held upright, or liquid will be propelled out the escape
hole.
(7) Use gloves for protection against the cold.
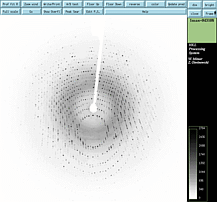
Mike (How to interpret a diffraction image).
(0) What is that shadow at the center of the image? beamstop.
(1)Where are the most intense diffraction spots? Near the beam stop.
(2) Why do the spots get weaker with higher diffraction angle?
While proteins are well ordered on a gross 20Angstrom level (low resolution),
on the 1.0Angstrom level (high resolution level), there are variations between
molecules. Vibrations, rotations of side chains, etc. The orderliness
breaks down on the sub-angstrom level.
(3) Why do we see spots, and not a continuous transform of light and dark
patches?
(4) Why are the spots arranged on concentric circles? There
is a geometrical construction that crystallographers use to help them rationalize
the observed pattern of spots. It illustrates (for a given orientation
of the crystal) which set of Bragg planes are in position to satisfy Bragg's
law. The construction is called Ewald's sphere of reflection. Imagine
the crystal is oriented at the center of a sphere with radius 1/wavelength.
There is a reciprocal lattice with its origin situated 1/wavlength
distance away where the direct x-ray beam exits the sphere. The lattice
has dimensions that are inversely proportional to the real crystal lattice.
At (h,k,l) integer increments along the lattice are reciprocal lattice
points. When these points touch Ewald's sphere, then the set of planes
hkl are in position to produce diffraction. Because these reciprocal
lattice points lay in planes, they intersect with the sphere as a circle.
Hence the diffraction pattern looks like concentric circles. The
lattice rotates with the crystal about its separate origin. So as the
crystal moves, the circles move accross the image. Show movie.
Notes for future: Make another illustation of Ewald's sphere with
rotated crystal. Show that reciprocal lattice tilts with crystal.
Use same picture the following week to illustrate indexing.
Ask students to index a spot in this picture.
|