0) Ordering should
be done around December 1
(a)Order PhastGel Homogeneous 12.5 get 2 packs
of 10. cat no. 17-0623-01.
- Do not use homogeneous 20. ProK runs too slow on
them.
- If you have to use homogeneous 20, then use 360 AVh to
run the gel.
(b) Order two bottles of proteinase K (100mg)
cat no. P2308 from Sigma.
1) Send e-mail to Yeates Lab (toygroup@mbi.ucla.edu) giving the date and
time of the lab.
- Ask permission to use their pipetmen and lab bench space.
- Ask them to stay out of the lab during this time.
- Post signs on the doors.
- Place a sign over the PhastSystem (right side) reserving
it.
- Put up reservation signs on lab door and the Phast system.
Print out
/data1/users/sawaya/HTML/m230d/Crystallization/reserved.cdr
Subject: a lab visit from Chem M230D
Hi everyone:
Duilio and I are planning to introduce crystallization techniques
to eight Chem M230D students in BH 269.
1) 2-4 PM Today (Tues Jan 9)
2) 2-4 PM Wednesday, January 10
We plan to use your bench space and pipetmen.
We will be running a Phast gel
and setting up crystal trays.
If you have any concerns, please talk to Duilio or me.
Thank you for your cooperation!
Mike
Thank you for your cooperation!
Mike
2) We usually have two lab sessions, each with 10 students.
For each session, you will need:
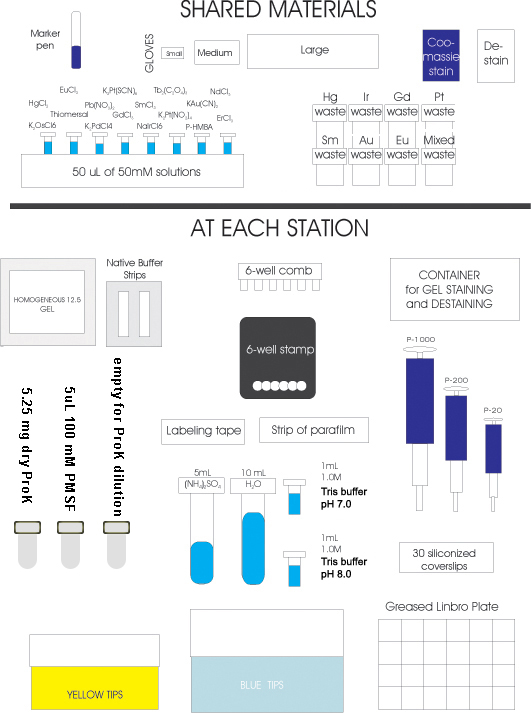
Inna's preparations:
- 6- VDX plates greased , place tape on the cover to be used
for a label (Inna)
- 180 -siliconized glass cover slips divided into six boxes
of 30 (Inna)
- 30 mL of 4M ammonium sulfate divided into six 5mL aliquots
(Inna)
- 6 mL of 1.0M Tris pH 7.0 and pH 8.0, each divided into six
1 mL aliquots (Inna)
- 60 mL of distilled water divided into six 10 mL aliquots
(Inna)
- 6 boxes of yellow tips (Inna)
- 6 boxes of blue tips (Inna)
- 6 sets of pipetmen P-1000, P-100, P-20 (Inna)
- Large, Medium & Small gloves. (Inna)
- Coomassie stain and destain.(Inna)
- Parafilm and black sample-stamp-wells(Inna)
- Six 500 uL eppendorf tubes in a rack.(Inna)
- Tip disposal containers for each type of heavy atom. Hg,
Sm, Au, Eu, Pt, Gd, Ir, Er.
- Need two complete sets, one set for each half of the
lab bench.
- 6 homogeneous 12.5 gels (Inna)
- 6 sample loaders (6-well combs) (Inna)
- 12 native buffer strips (Inna)
Mike's preparations
- label 6 500uL tubes with 5.25 mg proteinase K (Mike)
- label 6 500uL tubes with 100 mM PMSF (Mike)
- get 6 empty 500uL tubes for diluting proteinase K (Mike)
- make bench labels for groups A,B, and C. (Mike) /data1/users/sawaya/HTML/m230d/Crystallization/groupsigns.cdr
- Make 100mM PMSF by weighing 0.0174 g PMSF, dissove in 1mL
isopropanol. (Mike)
- Weigh 5.25 mg (0.00525g proteinase K). Students will dissolve in 70 uL water.
Use greater than 40 mg/mL or else you will get porcupine crystals. See
notebook3 page 5 and 81 and 79 and 66.
- Install the reverse polarity electrode in the PhastSystem
(Mike)
- reverse polarity electrode
3) Prepare a tray of native Proteinase K crystals 1 week before class.
4) Post an assignment sheet for data collection next week.
5) See your lecture notes on Page 4 of Notebook 3. See powerpoint
presentation /data6/users/sawaya/HTML/m230d/Crystallization/ppt/crystallintro_2007.ppt
Improve
them.
 |
