|
Perparing Iodide or Cesium derivatives
|
|
for a SIR, SAD, or SIRAS experiment
|
5-amino-2,4,6-triiodoisophthalic acid (I3C)
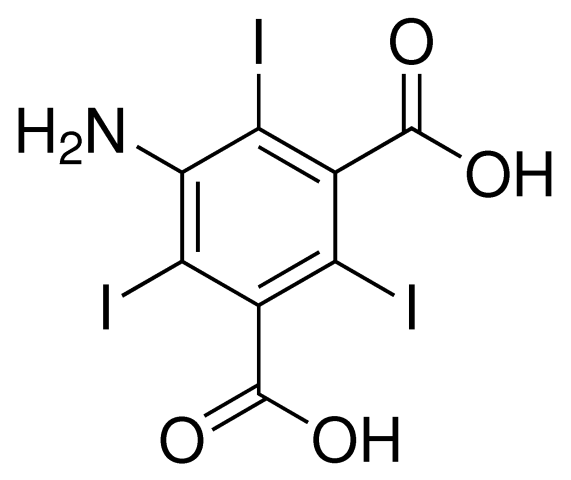
Adapted from Beck et al., 2008, A magic triangle for experimental phasing of macromolecules. Acta Cryst vol D64, 1179-1182. and Beck et al., 2009, How to get the magic triangle and the MAD triangle into your protein crystal. Acta Cryst vol F65, 1068-70.
I3C has the advantage of 3 iodide atoms per molecule for additional phasing power.
See Tobias Beck's web page for additional information.
(1) I3C is located in room 219A, in the drawer below the balance. The drawer is labeled "Heavy atom ends". In a small brown glass bottle with red lid.
It was ordered from Sigma http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/444367
(2) Weigh 0.03g I3C (30 mg)
(3) Dissolve I3C in 59 uL of 2.0 M lithium hydroxide. (Makes 1.0M I3C solution) (I3C=556 g/mol) as recommended by Tobias.
(4) Mix 3.5 microliters I3C solution with 6.5 microliters reservoir and 3.5 microliters 100% glycerol stock for cryoprotection. This makes approximately 260 mM I3C, final concentration. Tobias recommends up to 500mM concentration.
(more or less glycerol may be required for cryoprotection)
(5) Soak crystal 5 minutes in the cryo-I3C solution.
(6) Mount crystal on cold stream
As a positive control, I3C was used to derivatize proteinase K. You can download the XDS formatted structure factors or the Scalepack formatted structure factors and log file.
|
Vaporizing iodine labeleing (VIL) of tyrosine residues
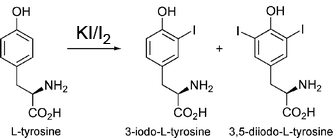
Adapted from Miyatake H, Hasegawa T, Yamano A. New methods to prepare iodinated derivatives
by vaporizing iodine labelling (VIL) and hydrogen peroxide VIL (HYPER-VIL). Acta
Crystallogr D Biol Crystallogr. 2006 Mar;62(Pt 3):280-9.:
(1) Find KI (potassium iodide) in room 269A, in the wooden cabinet near the shaker room, in a small white plastic bottle. Find I2 (elemental iodine) in the same room, in the blue cabinet near the hood, in a bin labeled "toxic and carcinogen", in a medium brown jar.
(2) Weigh 0.250 g KI in Eppendorf tube. (KI=166 g/mol)
(3) Dissolve KI in 1 mL of water.
(4) Weigh 0.135 g I2 in another Eppendorf tube. (I2 = 126.9 g/mol)
(5) Transfer the KI solution to the solid I2 to dissolve. (According to the paper, this makes 0.67 M KI/ 0.47 M I2, but by my calculations, this is 1.5 M KI/ 1.06 M I2 )
(6) Store at -20 deg C in 50 microliter aliquots
(7) Remove glass coverslip containing the drop with the crystal to be derivatized. Place 0.2 microliters on coverslip next to crystal drop, NOT touching the drop.
(8) Invert the coverslip over the reservoir as it was before you removed it.
(9) Incubate for 30 minutes to 24 hours. VIL derivative is ready!
(10) If you need to increase reactivity, add 1 microliter of 30% hydrogen peroxide on the coverslip, separate from the drops containing KI/I2 and the crystal. This is HYPER-VIL.
|
[Overview] ·[Facilities] ·
[People]
· [Services] ·[Lectures] ·
[BioLinks] ·
[Stats]
·[Search]
|

