UCLA Department of Chemistry and Biochemistry
153AH - Fall 2009 - Instructors: Todd Yeates, Duilio Cascio, Tobias Sayre
ADH: An enzyme for the oxidation of alcohol
by Chloe Siu
|
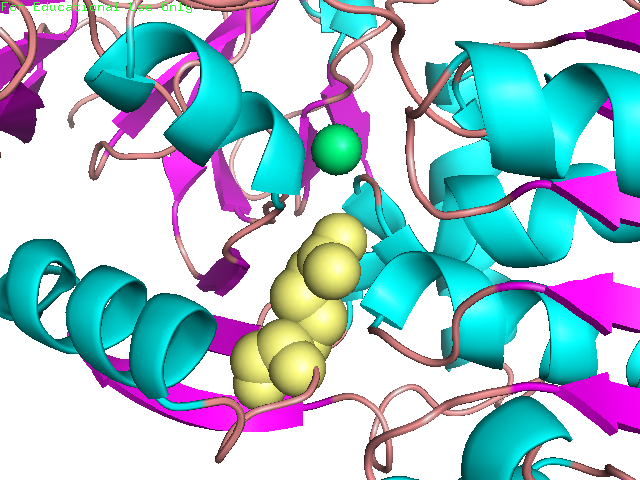
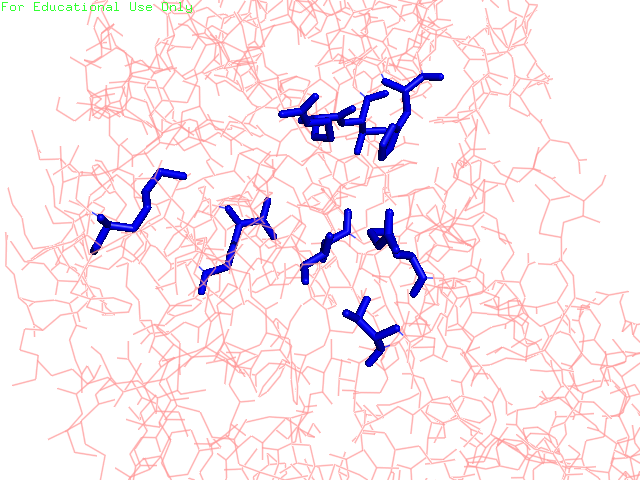
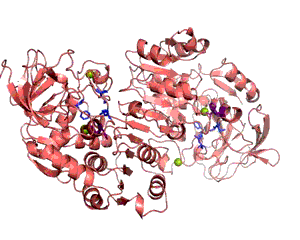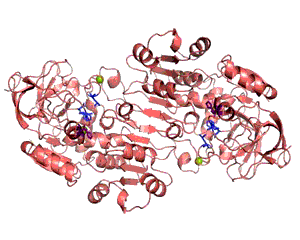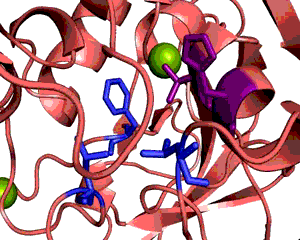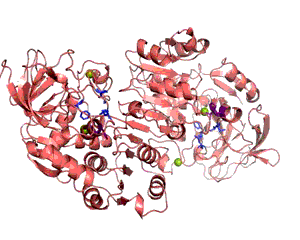
Enzymes provide proximity and proper orientation for substrates to interact. This lowers the activation energy of a chemical reaction, thereby accelerating its rate. Oxidoreductases constitute the largest class of enzymes. They catalyze the transfer of electrons from one molecule to another. Within this class of enzymes, hydrogen atoms and hydride ions are the most common groups transferred. Alcohol dehydrogenase (ADH) oxidizes alcohol into aldehyde or ketone through the reduction of NAD+ to NADH. In humans, human beta1 alcohol dehydrogenase is mainly used to convert ethanol into acetaldehyde. Human ADH also exists in beta2 and beta3 forms, in which cases Arg369 is replaced with histidine and cysteine, respectively (Figure 1). The beta1 isozyme is the most potent, party due to its strong interaction with the pyrophosphate group of the cofactor. Crystal structures reveal that the enzyme is dimeric and each ADH subunit has two zincs: one structural and one catalytic. (1, 3) The metal provides electrostatic stabilization to the complex by interacting with the alcohol oxygen, making the oxygen more nucleophilic and its proton more acidic. The catalytic zinc coordination involves five atoms: two sulfur atoms from Cys46, and Cys174, His67, a water molecule, and the alcohol oxygen. The human beta1 alcohol dehydrogenase also contains two other major domains: alcohol-binding domain and NAD+-binding domain. Besides interacting with the zinc, the substrate additionally binds to the enzyme via Phe93, Leu57, and Leu116. (2) This three-point attachment results in substrate stereo specificity. Finally, NAD+ binds to the enzyme via His51, Thr48, Arg47, Gly204, Val 203, Lys228, Thr274, Ile269, Arg271; these residues surround the NAD+ molecule. (2; Figure 2). Like many catalytic schemes, the reaction of Human Beta1 Alcohol Dehydrogenase involves a series of general base and general acid steps in order to transfer a hydride ion from the alcoholic substrate to a NAD+ molecule. In the first step, His51 accepts a proton from NAD+, which then accepts a proton from Thr48. This threonine, also bound to the alcohol and now negatively charged, deprotonates the alcohol. Since this oxidation is concerted, a hydride ion is eventually added to the pyridinic nitrogen in the NAD+ molecule, reducing it NADH. (2) The Human Alcohol Dehydrogenase facilitates the oxidation of alcohol by holding the reactants in close proximity and by supplying favorable interactions, in keeping with standard models for enzymatic catalysis. References (1) Buhler, et al (1984). Human alcohol dehydrogenase: structural differences between the beta and gamma subunits suggest parallel duplications in isoenzyme evolution and predominant expression of separate gene descendants in livers of different mammals. Proc Natl Acad Sci USA 81, 6320-24. (2) Hurley, et al. (1991). Structure of human β1β1 alcohol dehydrogensae: Catalytic effects of non-active-site substitutions. Proc Natl Acad Sci USA 88, 8149-53. (3) Davis, et al. (1996). X-ray Structure of Human β3β3 Alcohol Dehydrogenase J. Biol Chem. 271, 17057-61. (4) PDBID 1deh |
|