UCLA Department of Chemistry and Biochemistry
153AH - Fall 2009 - Instructors: Todd Yeates, Duilio Cascio, Tobias Sayre
Resistin: Disulfide-rich Hormone related to Obesity
by Amity Tung
|
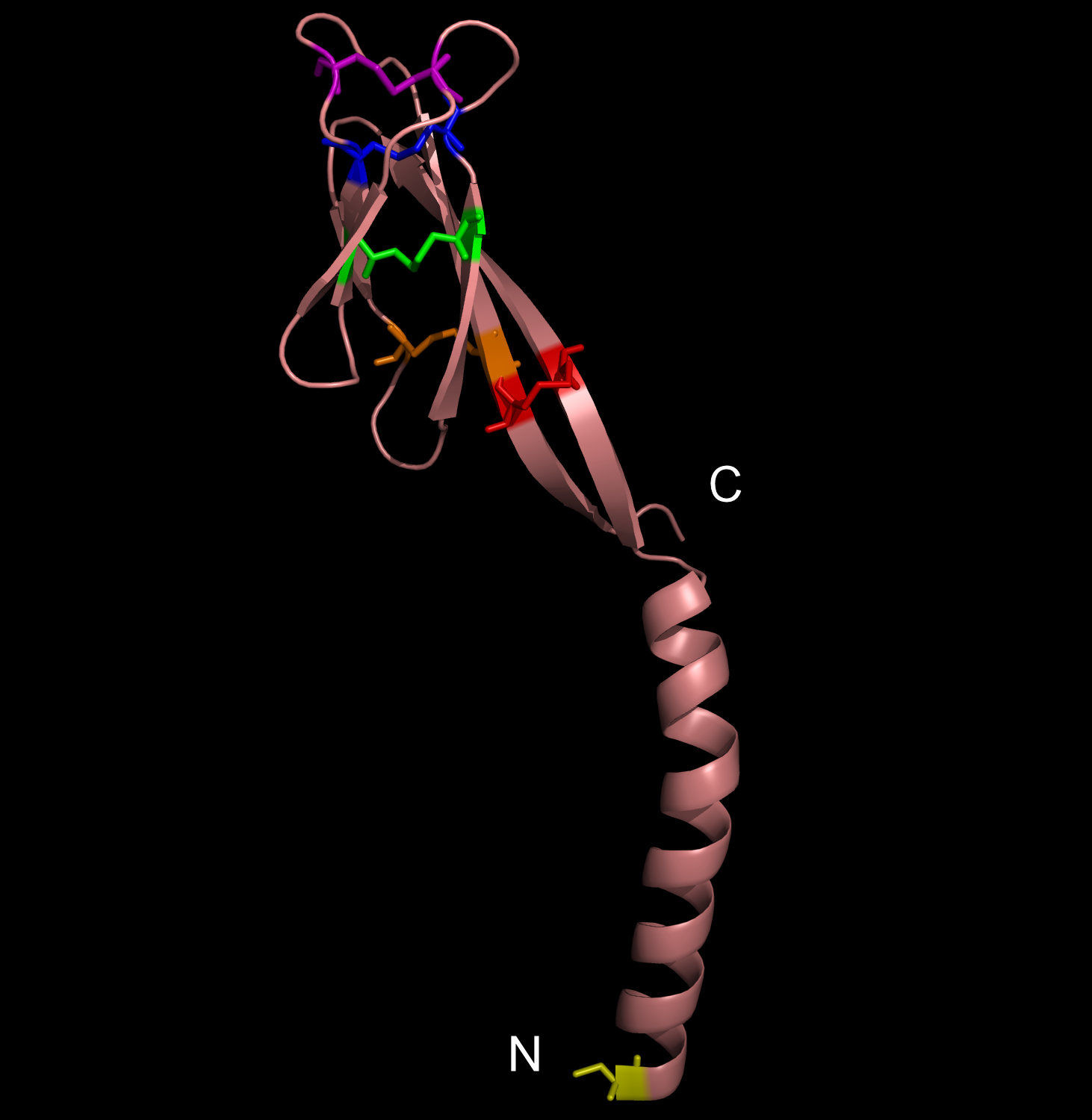
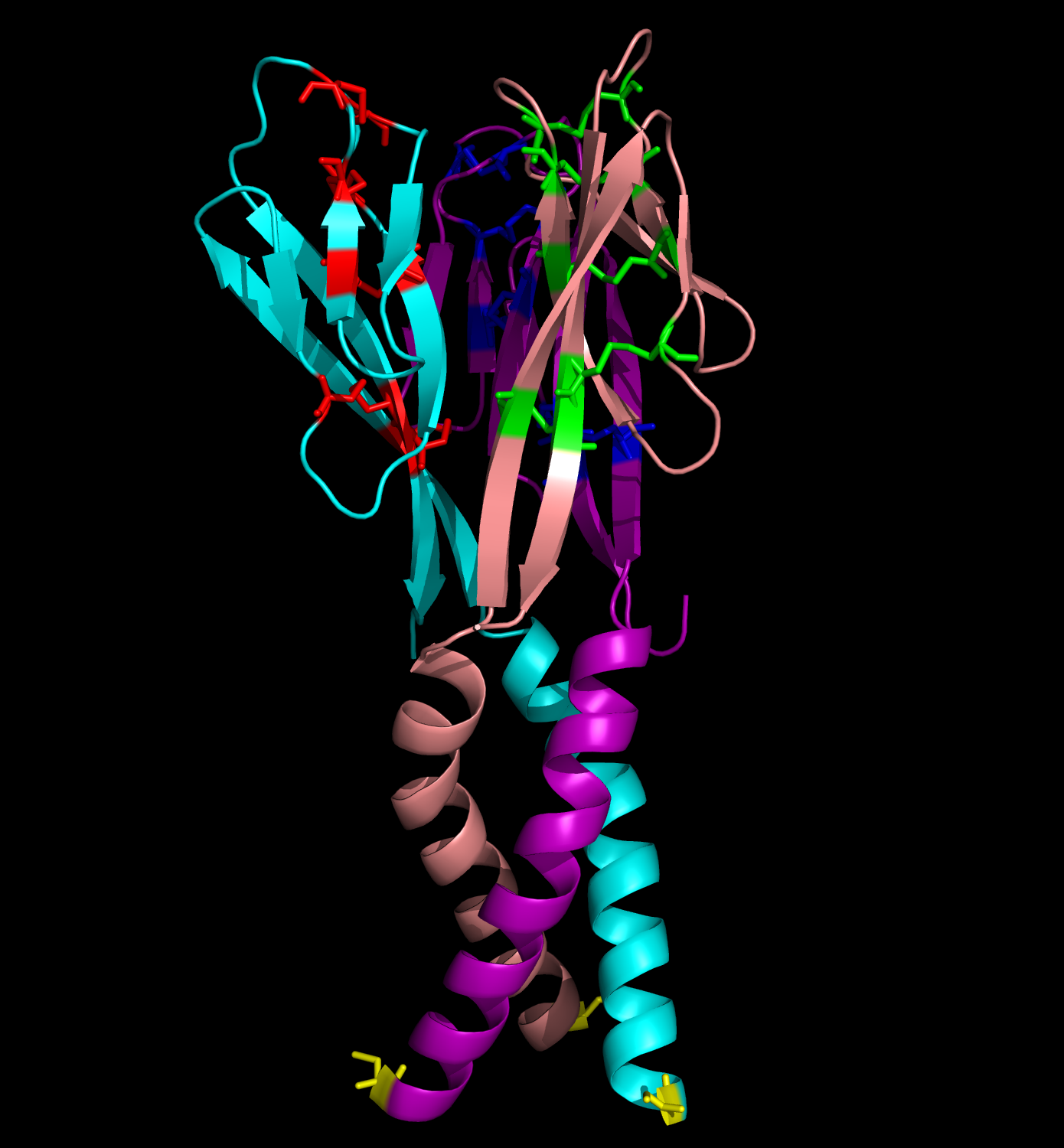
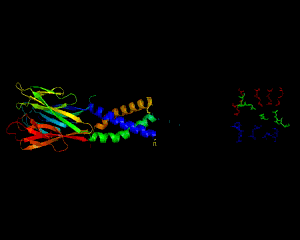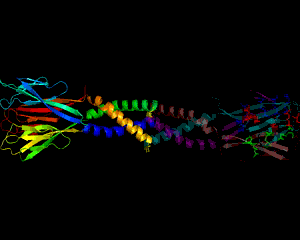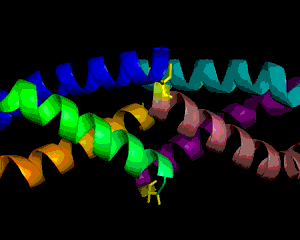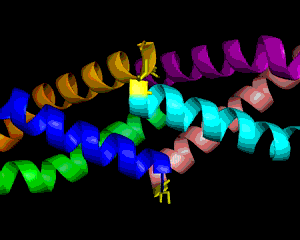
Obesity is increasing at an alarming rate in the US. Recent research indicates that approximately one-third of the adult population is obese, and that figure is still rising (1). Although it is believed that obesity leads to diseases such as Type II diabetes mellitus, not much is known about the link between obesity and insulin resistance. Nonetheless, some researchers suggest that resistin, the founding member of the resistin-like molecule (RELM) hormone family, can provide a molecular basis for this connection (2). Secreted from adipocytes, the cysteine-rich resistin is an insulin antagonist that causes insulin resistance, hence its name (3). Specifically, this peptide hormone lowers insulin sensitivity in the liver, increasing the rate of glucose production in the blood (3). The resistin monomer is 94 amino acids in length. Each monomer consists of a beta-sandwich head domain at the C-terminus, which is linked loosely to a helical tail at the N-terminus (Fig. 1). Whereas the N-terminal tail domain contains six turns of an alpha helix, the beta-head globular domain contains five disulfide bonds within two three-stranded antiparallel beta sheets, making it appear like a six-stranded jelly-roll. Three of these monomers position their N-terminal coiled coils parallel to each other, with side chain interactions in between, in order to form a trimer (Fig. 2). Resistin exists in two distinct states, the trimer and the hexamer, in approximately a 1:6 ratio. The predominant hexamer is essentially a homodimer of two identical trimers connected by disulfide bonds (Fig. 3). Although the trimer form exists independently in solution, the hexamer arrangement is favored due to the stability provided by the intertrimer disulfide bonds. Resistin self-assembles into a hexamer when the tails of each helix in one trimer connect to the clockwise-adjacent tails of the other trimer through Cys6 for three disulfide bonds (Fig. 3). Overall, each monomer has eleven cysteine residues, ten of which are conserved in the RELM family and form the disulfide bonds within the beta-head, and one of which is particular to resistin and forms the intertrimer disulfide bonds (2). The fact that 12% of the amino acids in resistin are cysteines attests to the importance of disulfide bonds in the hormone (3, 4). The hexamer and trimer forms are also known as the High Molecular Weight (HMW) and Low Molecular Weight (LMW) forms, respectively. Interestingly, the LMW trimer, which lacks intertrimer disulfide bonds, is found to be more biologically active, increasing insulin resistance and the corresponding hepatic glucose production (3). Thus, it is hypothesized that the free coiled-coil tails of the trimer are involved in receptor-binding interactions. That is, cleaving the critical disulfide bonds of the hexamer form in reducing conditions would activate the more potent trimer form of resistin (4). Therefore, control of disulfide bond formation and cleavage provides a mechanism to regulate the activity of resistin (3). Nonetheless, further studies are needed to better understand the link between obesity and diabetes. References (1)"Prevalence of overweight, obesity and extreme obesity among adults: United States, trends 1960-62 through 2005-2006." Cdc.gov. CDC, 2008. (2) Aruna, B., Ghosh, S., Singh, A.K., Mande, S.C., Srinivas, V., Chauhan, R., and N.Z. Ehtesham. (2003). Human recombinant resistin protein displays a tendency to aggregate by forming intermolecular disulfide linkages. Biochemistry 42, 10554-10559. (3) Patel, S.D., Rajala, M.W., Rossetti, L., Scherer, P.E., and L. Shapiro. (2004). Disulfide-dependent multimeric assembly of resistin family hormones. Science 304, 1154-1158. |
|