UCLA Department of Chemistry and Biochemistry
153AH - Fall 2009 - Instructors: Todd Yeates, Duilio Cascio, Tobias Sayre
TPx-B: An antioxidant from human blood cells
by Yuqi Wang
|
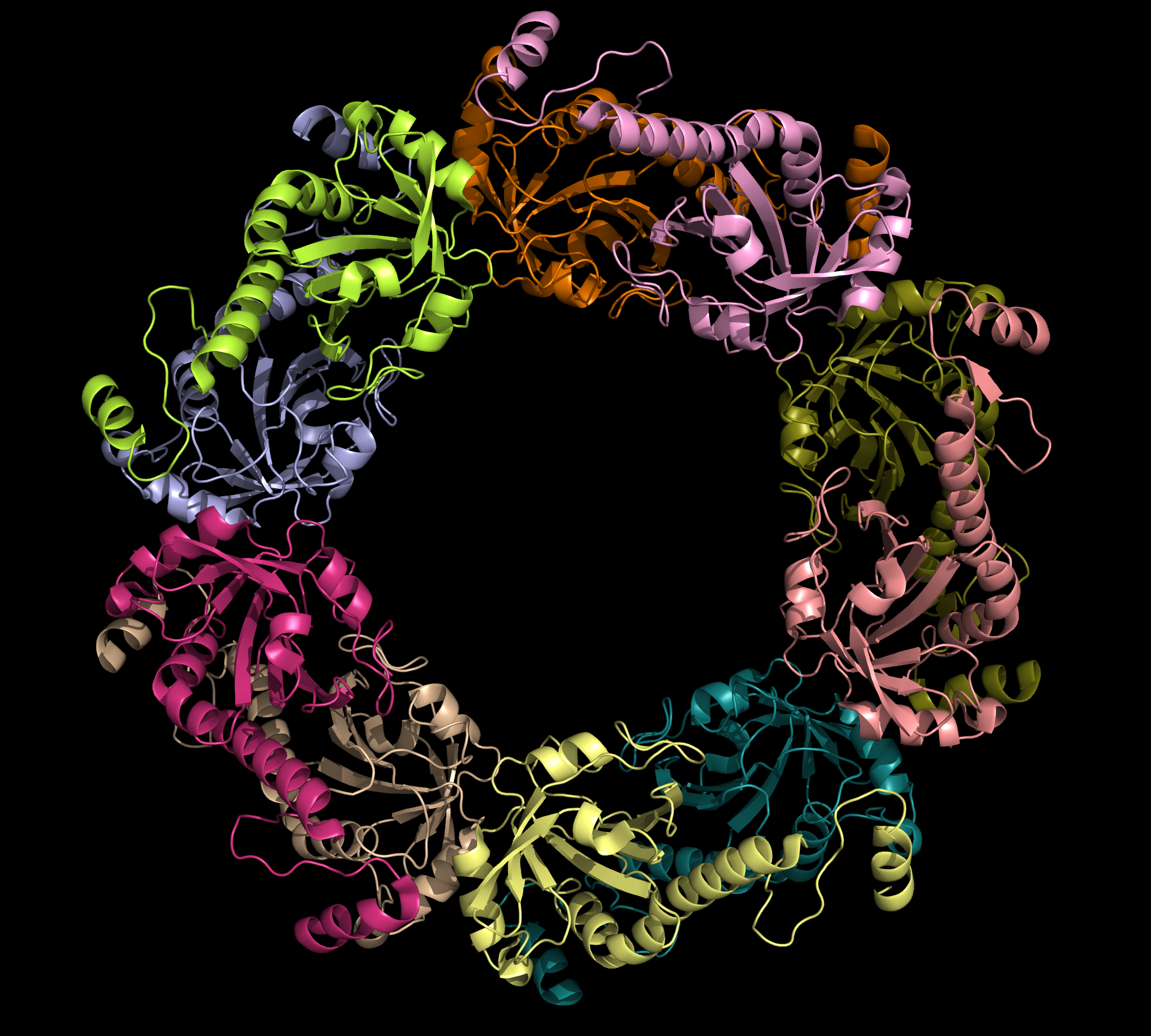
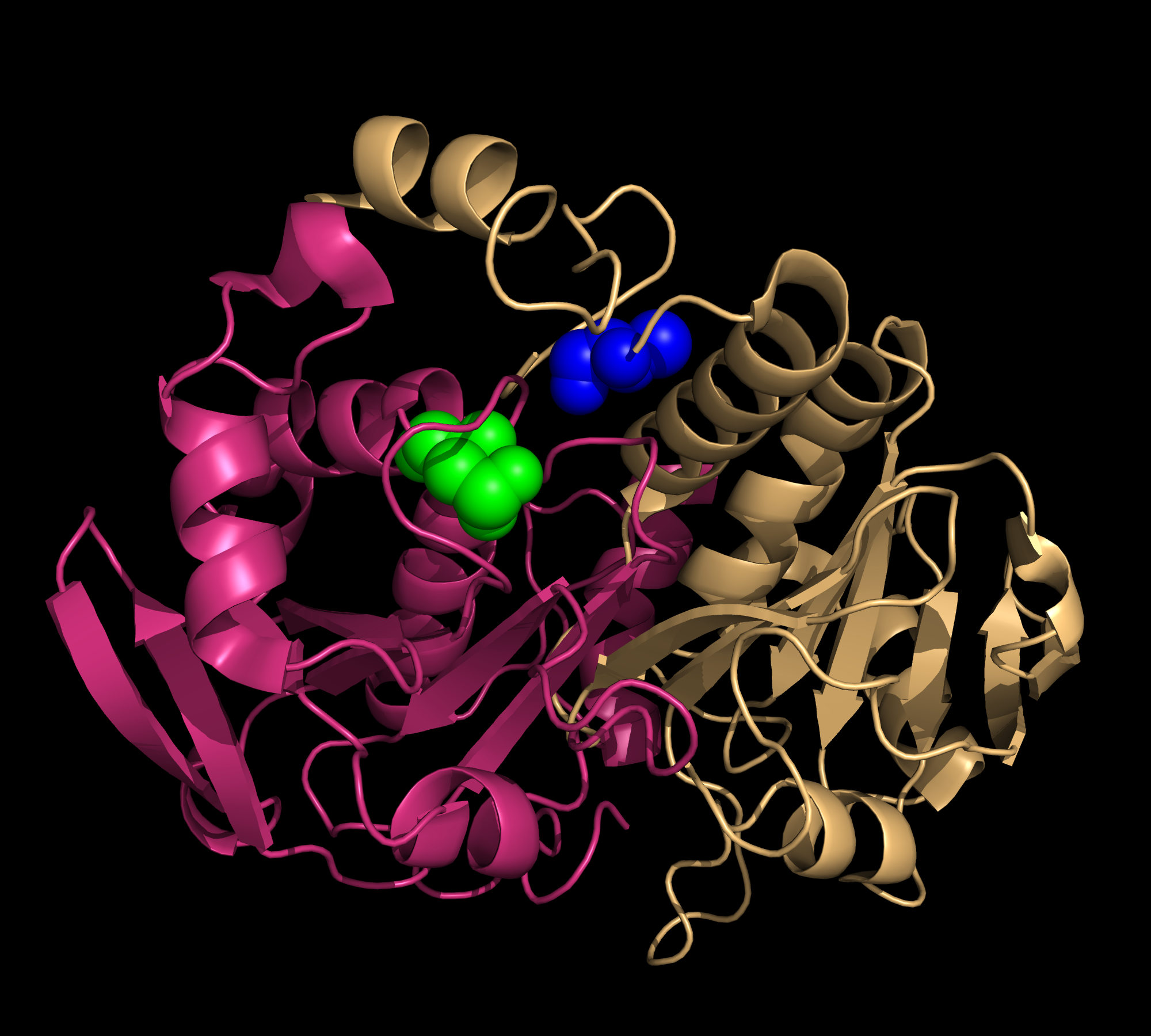
Peroxiredoxin is a family of thiol-specific antioxidants that catalyze the reduction of hydrogen peroxide and organic peroxide into water or corresponding alcohols, as in: 2 R'SH + ROOH = R'-S-S--R' + H2O + ROH. Although peroxiredoxins do not have catalytic activities as high as other typical antioxidant enzymes (e.g. glutathione-dependent peroxidases and catalases), they are universally expressed at high levels. This is consistent with their critical roles in biological metabolism (1). In humans, six peroxiredoxin isomers are currently known. They can be divided into three major subclasses: typical 2-cysteine Prdx, atypical 2-Cys Prdx, and 1-Cys Prdx . Thioredoxin peroxidase B (TPx-B), also known as peroxirecoxin-2, is a typical 2-Cys Prdx. It is the third most abundant protein in the human erythrocyte. The crystalline structure of this decameric protein has been determined to 1.7 Å resolution. It is composed of five dimers (198 amino acids in each monomer), linked end to end primarily via hydrophobic interactions, forming a shape of toroid (Fig.1). This decamer is proposed to represent an intermediate in the peroxidase reaction cycle (2). All peroxiredoxins carry out a two-step peroxidase reaction at a conserved, fully folded active site, centered around a redox-active cysteine called the peroxidatic cysteine (Fig.2). The first step of the reaction is common among the three peroxiredoxin classes. During this step, the peroxidatic cysteine attacks the peroxide substrate and is oxidized into sulfenic acid. The second step of the reaction involves the regeneration of a cysteine thiol group from sulfenic acid; this step distinguishes the three enzyme classes (1). During the regeneration step of cysteine thiol for typical 2-Cys Prxs, the sulfenic acid from one subunit is attacked by the resolving cysteine located in the C-terminus of a second subunit (Fig. 2). In the case of Trx-B, the redox-active Cys-51 is first oxidized to Cys-SOH (cysteine sulphinic acid). Then, Cys-SOH rapidly reacts with Cys-172-SH (approximately 10 Å away from Cys-51) from another subunit to form an intermolecular disulfide bond within a homodimer (Fig.3). The enzyme may be regenerated as a result of the reduction of the disulfide by one of several thiol-containing reductants Rí-SH (e.g. thioredoxin, AhpF). This regeneration marks the completion of one catalytic cycle (1). One conclusion drawn from this mechanism is that the oxidation of Cys51 appears to have trapped the structure in a stable decamer. This stabilized form occurs under oxidative stress. It also implies that the catalytic cycle of 2-Cys Prdx requires significant conformational changes. Similar decameric structures of TPx-B have been observed under electron microscopy, showing an association between the protein and erythrocyte membrane (2). References (1) Hall, et al. (2009). Typical 2-Cys peroxiredoxins--structures, mechanisms and functions. FEBS Journal 276, 2469-2477. (2) Schroder, et al. (2000). Crystal structure of decameric 2-Cys peroxiredoxin from human erythrocytes at 1.7 Å resolution. Structure 8, 605-615. (3) PDBID 1qmv |
|