Updated Oct 2001
1. Before this procedure, the following reagents should be ready: Towbin transfer buffer1, 5x working sample buffer solution2, PVDF membrane stain3, and PVDF membrane destain4.
2. Prepare protein loading sample using 8ul protein sample + 2ul 5x working sample buffer solution. Incubate sample at 37oC for 15 min. (Note: the minimum amount of protein for sequencing is 10 picomoles)
3. Spin down protein loading sample at max speed for 5 min.
4. Electrophores protein using the appropriate SDS-PAA gel. Use the 6/4-applicator (comb) and try to leave at least one lane between the standard marker and protein sample lanes.
5. When the electrophoresis is about to finish, prepare a container of 50mL of Towbin transfer buffer (two if there are two gels) for the gel(s), a box of 50mL of methanol for the PVDF membrane(s), and another container of 50mL of Towbin transfer buffer for filter papers.
6. When the electrophoresis is finished and before slicing the gel, immerse the PVDF membrane in 100% methanol for a few seconds until the whole membrane is translucent to wet the membrane.
7. Transfer the pre-wetted PVDF membrane to the box of Towbin transfer buffer. Also soak two filter papers per gel into another box of Towbin transfer buffer.
8. Slice the gel and soak it into the box where the PVDF membrane is for 3 min.
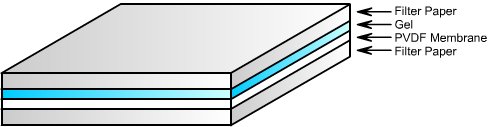
9. Sandwich the gel, PVDF membrane, & the filter papers as follow:

10. Roll over the sandwiched gel using a glass tube to avoid trapped bubbles.
11. Blot the protein using the settings: 15V (volt constant) for 15 min.
12. Stain the PVDF membrane by gently shaking the membrane in a box of PVDF membrane stain solution for a maximum of 5 min.
13. Discard the stain solution and soak the membrane in PVDF membrane destain solution and shake gently for 5 min. Then change the destain solution and shake for another 20 min.
14. Seal the membrane in between plastic films.
15. Send the membrane to the appropriate facility for analysis.
Solution Preparation (Note: filter the following gel solutions except #2 with 0.45-micron filter & store at 4oC.)
1. Towbin transfer buffer
2. 5x working sample buffer solution
3. PVDF membrane stain
4. PVDF membrane destain
5. 5x stock sample buffer solution
25mM Tris (30.3g in 1L)
192M glycine (14.4g in 1L)
20% methanol (200mL in 1L)
200ul 5x stock sample buffer solution5
10ul bromophenol blue solution (0.05% w/v)
10ul b-mercaptoethanol
0.025% CoomassieŽ Blue R-250 dissolved in 40% MeOH solution
50% MeOH solution
0.5M sucrose (42.78g)
15% SDS (37.5g)
312.5mM Tris (9.5g)
10mM Na2EDTA (0.925g)
Make to a volume of 225mL with distilled water. Heat gently to get into solution and adjust to pH 6.9 with 1N HCl. Adjust to a final volume of 250mL. Store at 4oC in 50mL aliquots.