UCLA Department of Chemistry and Biochemistry
153AH - Fall 2009 - Instructors: Todd Yeates, Duilio Cascio, Tobias Sayre
Leptin: An OB-encoded adipose derived hormone
by Jeffery Brumbaugh
|
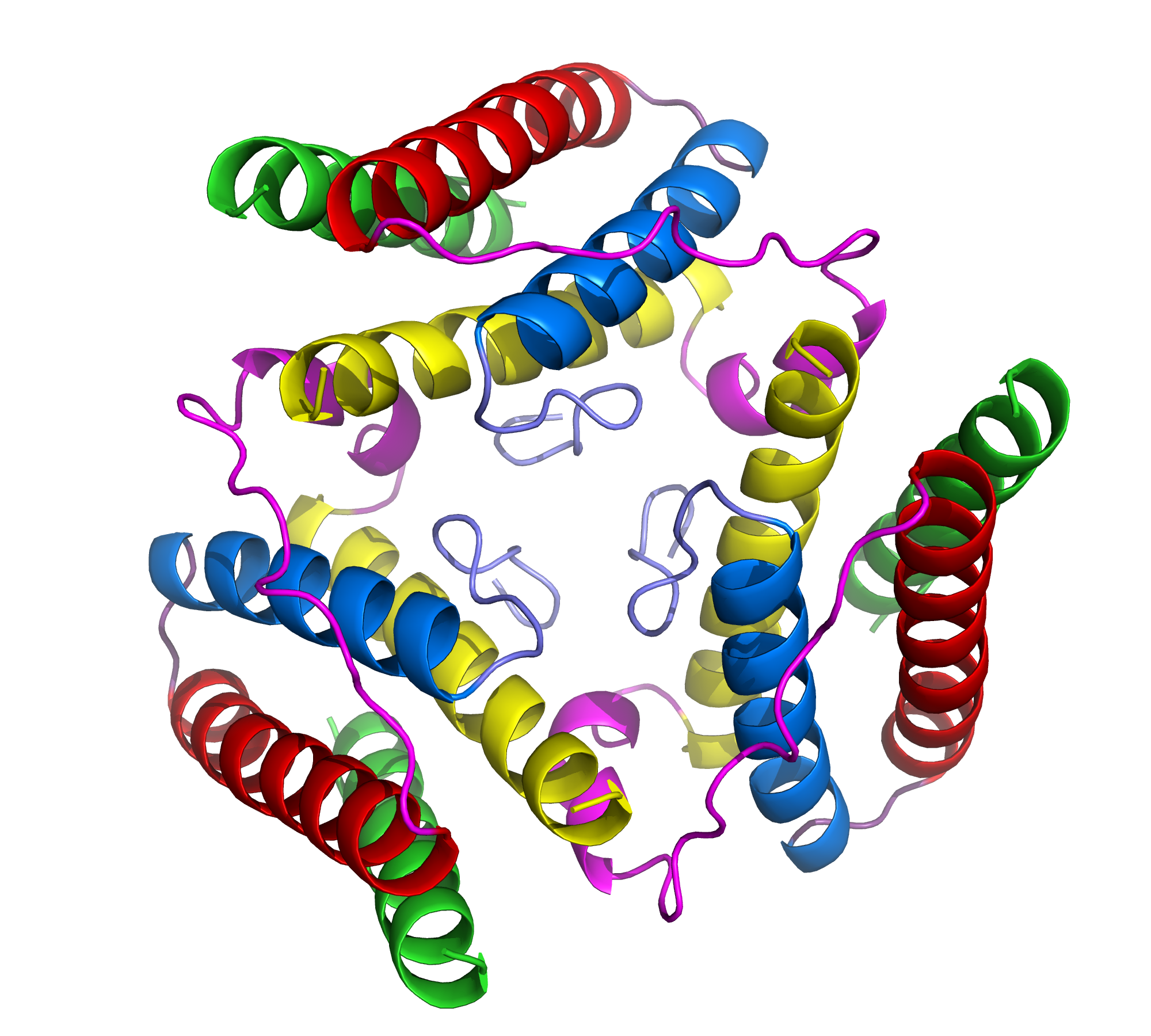
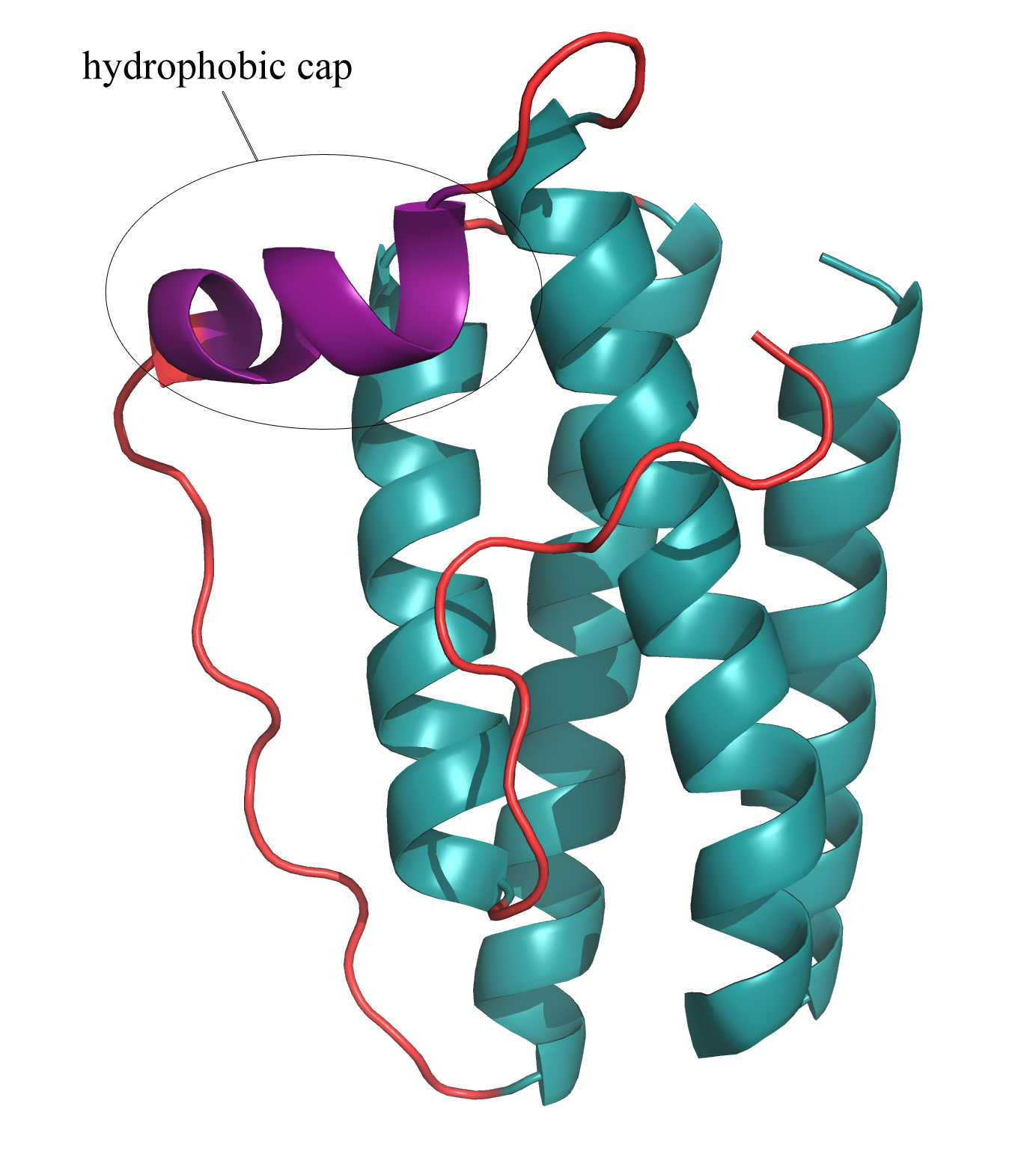
Cytokines represent a diverse family of substances, secreted in the immune system, that carry signals locally between cells. Consisting of proteins, peptides, and glycoproteins, cytokines play an extensive role in intercellular communication. One important subgroup of cytokines includes the adipose derived hormones secreted in the body by adipose (or fat) tissue. Leptin, a product of the obese gene (OB), is perhaps the best-known and most important adipose derived hormone (1). It is responsible for the regulation of energy intake and expenditure, appetite, and metabolism. An imbalance of leptin in the central nervous system (CNS) often leads to obesity (1). Appropriate levels of leptin must be present in the CNS in order for it to function correctly as a weight-lowering hormone. An imbalance of leptin is often the result of an inability to transport the protein to the CNS, rather than the result of mutations in the protein sequence (1). Accordingly, serum leptin concentrations are often elevated in obese individuals, when the protein cannot be properly transported to the CNS. Understanding the structure of leptin may lead to the design of more potent drugs that could act in place of leptin in leptin-resistant individuals (1). In its hexagonal crystal form, leptin packs as a monomer that consists of a four α-helix bundle (1). Four antiparallel α-helices are connected by two long crossover links and one short loop, resulting in a left-hand twisted helical bundle (1)(Fig. 2). The four α-helices take on an up-up-down-down folding pattern, which forms two layers of antiparallel helices and creates a hydrophobic core, which minimizes overall protein hydrophobicity (1)(Fig. 3). In its biologically active state, leptin assumes a trimer, with each helical bundle lying in slightly skewed planes relative to each other (Fig. 1). As a cytokine, leptin is extremely specific towards its receptors, and it has several highly conserved regions that enable this specificity. Variations in these regions would render the protein biologically inactive since it would no longer be able to fold correctly and bind the appropriate receptors. One such conserved region includes a small helical segment found in one of the interconnecting crossover loops (1)(Fig. 2). This tiny helix serves as a "hydrophobic cap" to bury the lipophilic residues on half of the helical bundle (1). Another highly conserved region of leptin includes the hydrophobic core of the helical bundle, as depicted in Figure 3. Finally, three of the four individual α-helices contain pronounced kinks in the middle that maximize the close contact between the helices in the bundle (1). All of these features are part of an intrinsic structural motif in leptin that is vital in providing the cytokine with its receptor-recognition properties. A thorough analysis of the structure of leptin is key in understanding its full role in OB-related diseases, such as obesity and diabetes, and in designing potential pharmaceutical analogs. References (1) Zhang F, et al. (1997). Crystal structure of the obese protein leptin-E100. Nature 387, 206-209. (2) PDBID 1AX8 |
|