UCLA Department of Chemistry and Biochemistry
153AH - Fall 2009 - Instructors: Todd Yeates, Duilio Cascio, Tobias Sayre
TERT- Catalytic subunit of telomerase
by Dylan Goodrich
|
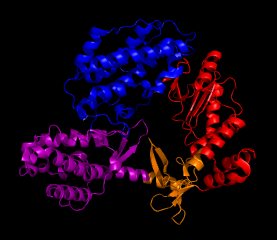
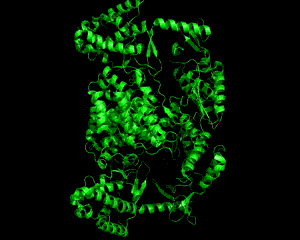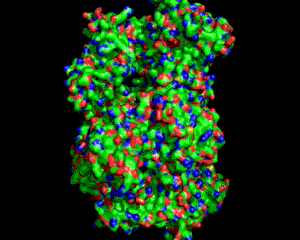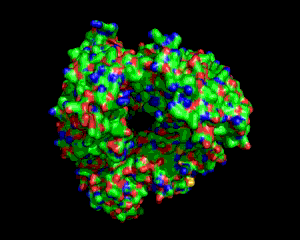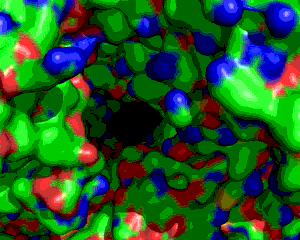
Telomeres are nucleotide caps on DNA that protect the edges of the strand and maintain chromosomal integrity. However, through frequent cellular divisions, telomeres lose length and at a certain point the cell becomes senescent, or unable to divide any further. Telomerase (TER) is an enzyme that catalytically lengthens telomeres early in life, but discontinues functioning in adulthood. This loss of activity leads to aging properties. It has been discovered that a key factor in the reproductive success of cancerous cells is the reactivation of telomerase, which allows for continual cell division within the host. The catalytic subunit TERT in telomerase is a protein that combines with an RNA component TER, and through this association allows repeated, processive addition (a unique feature of telomerase) when adding nucleotides to the 3' end of a DNA substrate. Its closest homologues are reverse transcriptases (as in HIV), which serve to create viral DNA from RNA templates using a similar mechanism. The TERT subunit that is discussed and pictured is from the telomerase enzyme of Triboleum castaneum, the Red Flour Beetle, and was the first defined structure of telomerase(1). The N terminus and C terminus motifs of TERT are adjacent to one another, forming a ring structure that is about 26 Å long and 21 Å wide. This arrangement is suitable to fit a double-stranded nucleic acid up to 8 bases long. Within the ring the different motifs that surround the center are designed for separate functions during catalysis (Fig. 1). The TRBD domain (purple) is mostly helical and binds the RNA while the Palm (red) and Fingers (orange) motifs combine to form the reverse transcriptase domain that binds the DNA and contains the active site for telomere elongation. Three aspartic acid residues constitute the active site of the enzyme. A closer look at these two domains (Fig. 2) shows the active site and RNA binding loop in close proximity to one another, and taking advantage of the ring structure. Elongation occurs by the formation of a nucleic acid heteroduplex at the active site with DNA and RNA (1). TERT can act as both a monomer and a dimer. The dimer configuration retains a ring structure like its monomeric form (Fig. 3) which allows it to function in the same way. The interior of the ring is spiraled in a way that resembles the backbone of DNA. Residues of lysine and asparagine that are found within the spiral contribute to DNA placement and facilitate the nucleic acid heteroduplex by aligning the 3' end of the DNA towards the active site. Future work with this enzyme will be to identify the human form of telomerase and research ways to inhibit activation and prevent cancer proliferation. References (1) Gillis, et al. (2008). Structure of the tribolium castaneum telomerase catalytic subunit tert. Nature 455, 633-638. (2) PDBID 3du6 |
|