UCLA Department of Chemistry and Biochemistry
153AH - Fall 2009 - Instructors: Todd Yeates, Duilio Cascio, Tobias Sayre
The Sodium-Potassium Pump: Na+,K+-ATPase
by Charisma Urbiztondo
|
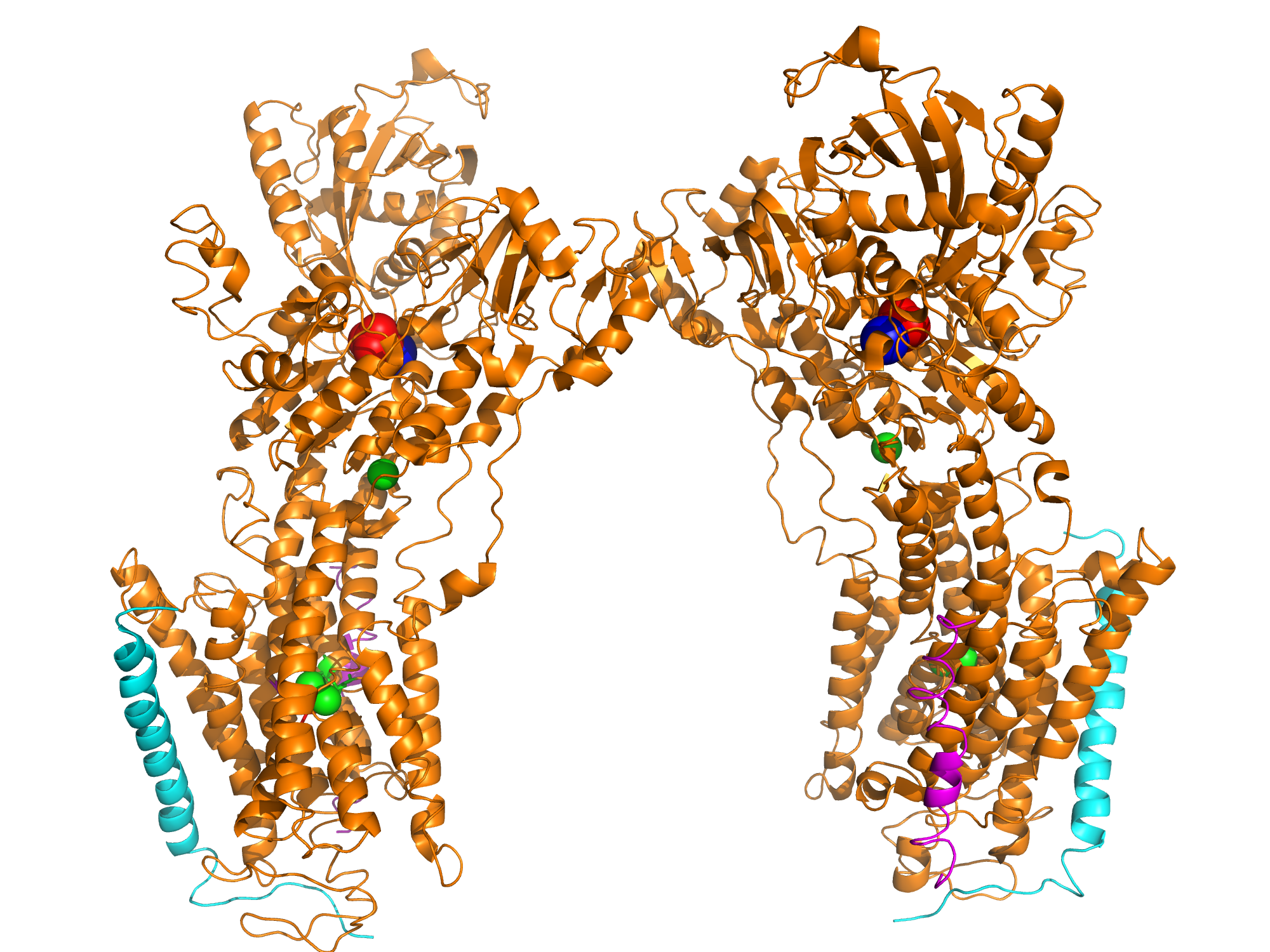
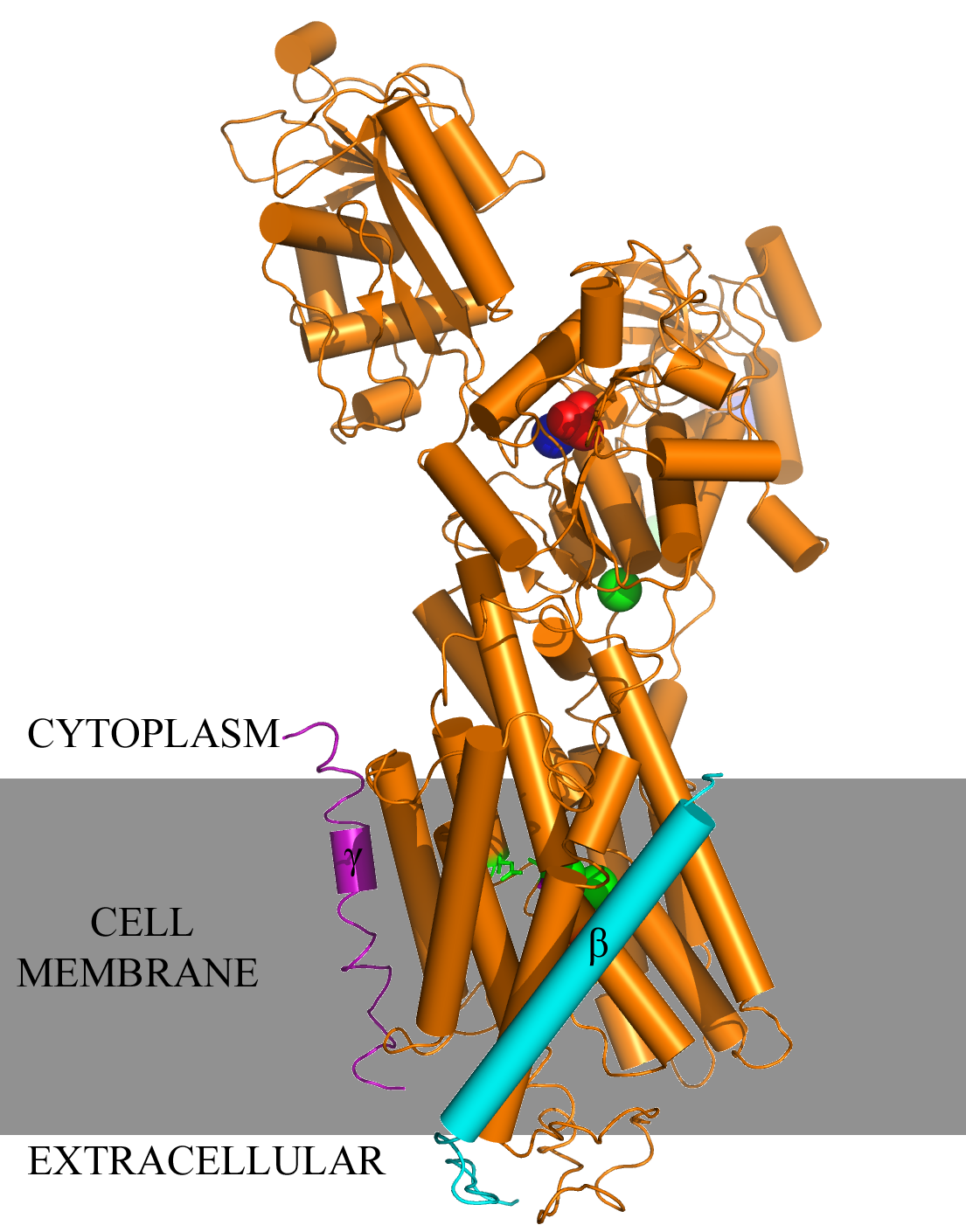
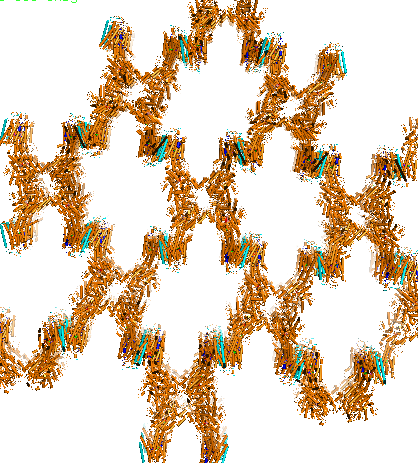
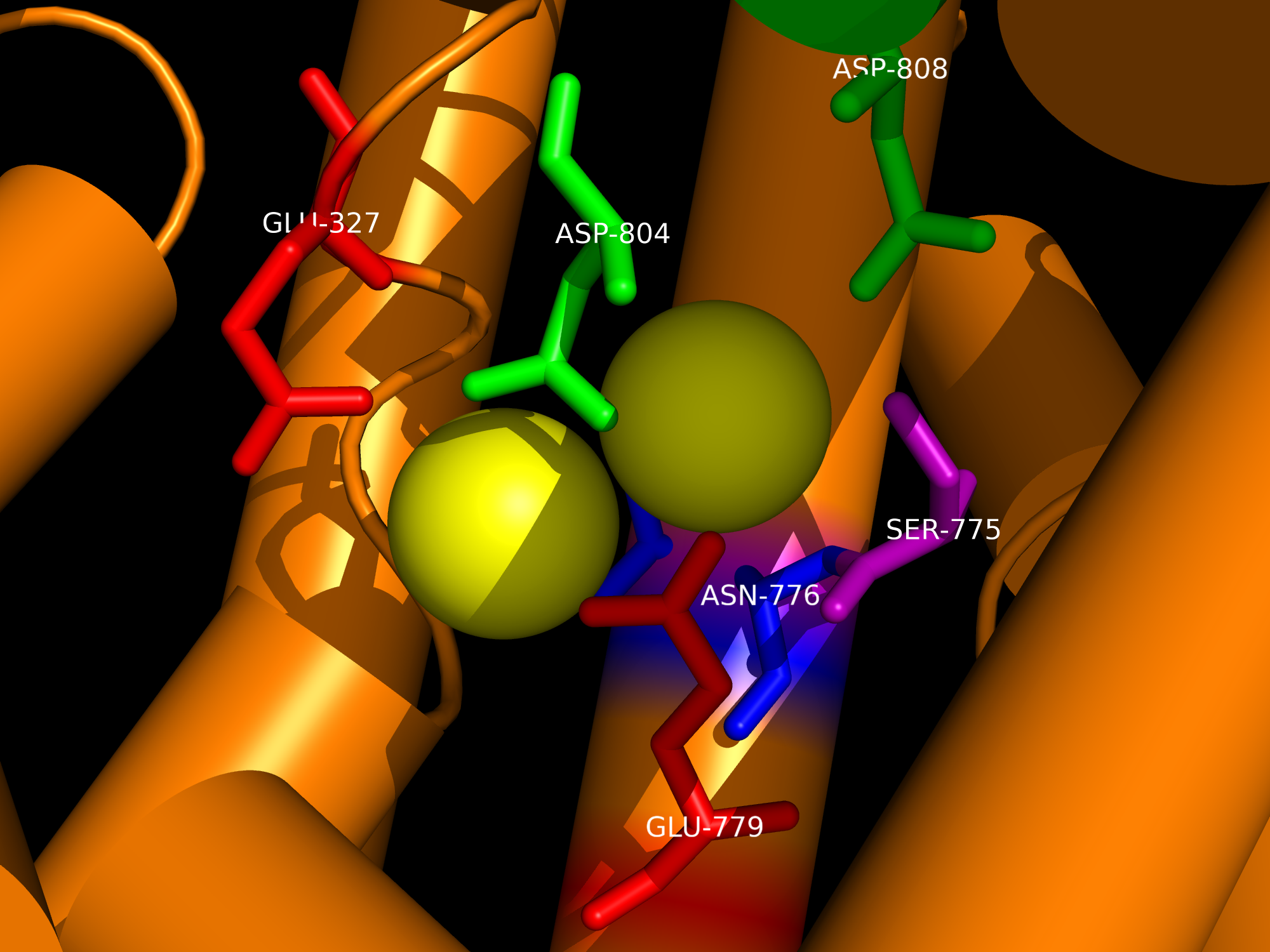
Charged ions, carbohydrates, and other molecules are often too polar or too large to penetrate the nonpolar cell membrane and are therefore transported using various transmembrane channels. An example is the Na+,K+-ATPase transmembrane protein, which uses active transport to move sodium and potassium ions in opposite directions across the cell membrane (1). An electrochemical gradient is produced by transporting three sodium ions out of the cell and two potassium ions in for each ATP hydrolyzed. The generation of membrane potentials is essential for animal cells, particularly for muscle cell contraction and neuronal cell restoration, as well as for pH maintenance and cellular ion uptake in other cell types. The α, β, and γ subunits of the Na+,K+-ATPase primarily consist of α-helical structures. The αβγ assembly forms a transmembrane protein complex (Figure 1b). In the α-helices, the outer residues facing the membrane are comprised mostly of aromatic and nonpolar side chains, and are presumed to interacts with the highly hydrophobic lipid bilayer. Oriented in a head-to-head manner, the cytoplasmic region of the α subunit participates in intermolecular interactions with α subunits of other Na+,K+-ATPases, forming arrays of molecules within the membrane (Figure 2). Categorized as a P-type ATPase, the pump must hydrolyze ATP in order to generate the necessary membrane potential (1). ATP involvement allows the protein to undergo conformational changes that enable selective binding of ions, resulting in a more effective delivery of sodium and potassium ions into the cytoplasm or the extracellular matrix. The original conformation of the Na+,K+ pump exhibits a high affinity for sodium ions; three Na+ initially bind to the protein. ATP then phosphorylates the central cytoplasmic loop of the Na+,K+-ATPase α-subunit and supplies the required energy to change the shape of the pump, thus coupling driving delivery of the Na+ ions out of the cell (1, 2). The new Na+,K+-ATPase form (with the phosphate still bound to the ATP-binding pocket) highly favors the binding of two potassium ions present in the extracellular matrix. Glutamate and aspartate residues provide carboxyl side chains that present oxygen ligands for K+ binding and occlusion (Figure 3) (1). Furthermore, serine and asparagine residues are also believed to contribute to the K+ binding site. Dephosphorylation of the central cytoplasmic loop occurs after binding of potassium ions, causing the pump to revert back to its original conformation, thus releasing the bound K+ ions into the cytoplasm. The amino acid residues directly involved in cation binding within the helices of the α subunit have yet to be precisely identified and are the subject of further study. References (1) Morth et al. (2007) Crystal structure of the sodium-potassium pump. Nat. Rev. Microbiol. 450, 1043-1049. 2) Gatto et al. (1999) Cys577 Is a Conformationally Mobile Residue in the ATP-binding Domain of the Na,K-ATPase &apha;-Subunit. J. Biol. Chem. 274, 24995-25003. (3) PDBID 3b8e |
|