UCLA Department of Chemistry and Biochemistry
153AH - Fall 2009 - Instructors: Todd Yeates, Duilio Cascio, Tobias Sayre
The Flexibility of Drug Inhibition in HIV-1 Reverse Transcriptase
by Janahan (Jovo) Vijanderan
|
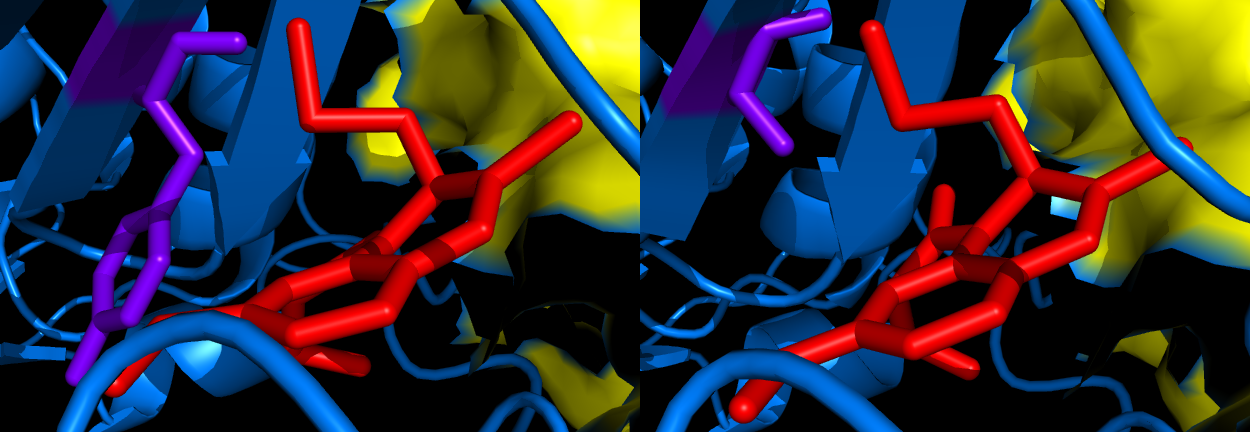
Human Immunodeficiency Virus (HIV) is a retrovirus that causes acquired immunodeficiency syndrome (AIDS), a condition in which the immune system becomes extremely fragile and begins to fail. There are two types of HIV, HIV-1 and the less prevalent HIV-2. To date, it has been very difficult to find a working mechanistic cure to HIV because reverse transcription (synthesizing DNA from RNA) is a required step in the life cycle of the HIV virus (2). HIV's mechanism of reverse transcription is what makes it so deadly. Targeting reverse transcriptase (RT) is therefore a central focus in development of chemotherapy against HIV infection. These efforts have been enabled by structural studies of HIV-1 RT, which have shown that it is an asymmetric heterodimer. (Fig 1) Several potent and extremely specific non-nucleoside RT inhibitors (NNRTIs) of HIV-1 have been discovered. The Hoecsht-Bayer inhibitor, HBY 097 ((S)-4-iso-propoxycarbonyl-6methoxy-3-methyliomehtyl-3,4-dihydroquinoxalin-2(1H)-thione), is extremely potent in inhibiting HIV-1 replication in vitro (1). The mode of binding of HBY 097 to HIV-1 RT is significantly different from the "butterfly-like" arrangement seen in many other non-nucleoside inhibitors (NNRTIs) (1). The main difference between the Tyr188Leu HIV-1 RT Mutant/HBY 097 complex and the wild type RT/HBY 097 complex include repositioning and conformational changes in the inhibitor-binding pocket (Fig 2). The Tyr188Leu HIV-1 RT mutant is not inhibited as well by the HBY 097 complex due to the loss of these important protein-inhibitor interactions, specifically involving the aromatic ring of Tyr 188 (1). Research has shown that the loss of this binding energy is partially overcome by additional contacts formed to nearby residues following conformational changes in the inhibitor. This key finding suggests that inhibitor flexibility can minimize drug resistance. The Tyr188Leu mutant HIV-1RT is 15-25 times more resistant to inhibition by HBY 097 than its wild-type counterpart (1). The reason for this is that HBY 097 inhibitor geometry and binding differs between the two strains of RT. A specific example is the hydrogen bond between the carbonyl O of Lys 101 and the N2 of HBY 097, which is shorter in the mutant complex. Furthermore, the interplanar angle between the two "wings" of the arrangement is 5 Å smaller in the mutant, thus reducing the contacts between HBY 097 and Leu100. There are also differences in the Non Nucleoside Inhibitor Binding Pocket (NNIBP) between the wild-type and mutant. The most significant change is a repositioning of the Phe227 side-chain, which occupies a position in the mutant that would cause sterically unfavorable contacts in the wild-type structure. Furthermore there is an expansion of the NNIBP in the mutant structure that results in the change of inhibitor geometry and consequentially explains the lesser potency of the HBY 097 drug with the mutant enzyme. References (1) Yu, et al. (1998). Structures of Tyr188Leu Mutant and Wild-Type HIV-1 Reverse Transcriptase Complexed with the Non-nucleoside Inhibitor HBY 097: Inhibitor Flexibility is a Useful Design Feature for Reducing Drug Resistance Protein-based organelles in bacteria: carboxysomes and related microcompartments. J. Mol. Biol.K 284, 313-323 (2) Hartwell, et al. (2008) Genetics: From Genes to Genomes. McGraw-Hill, New York, New York. Pgs 220-221 (3) PDBID: 1bqm (mutant) (4) PDBID: 1bqn (wild-type) |
|