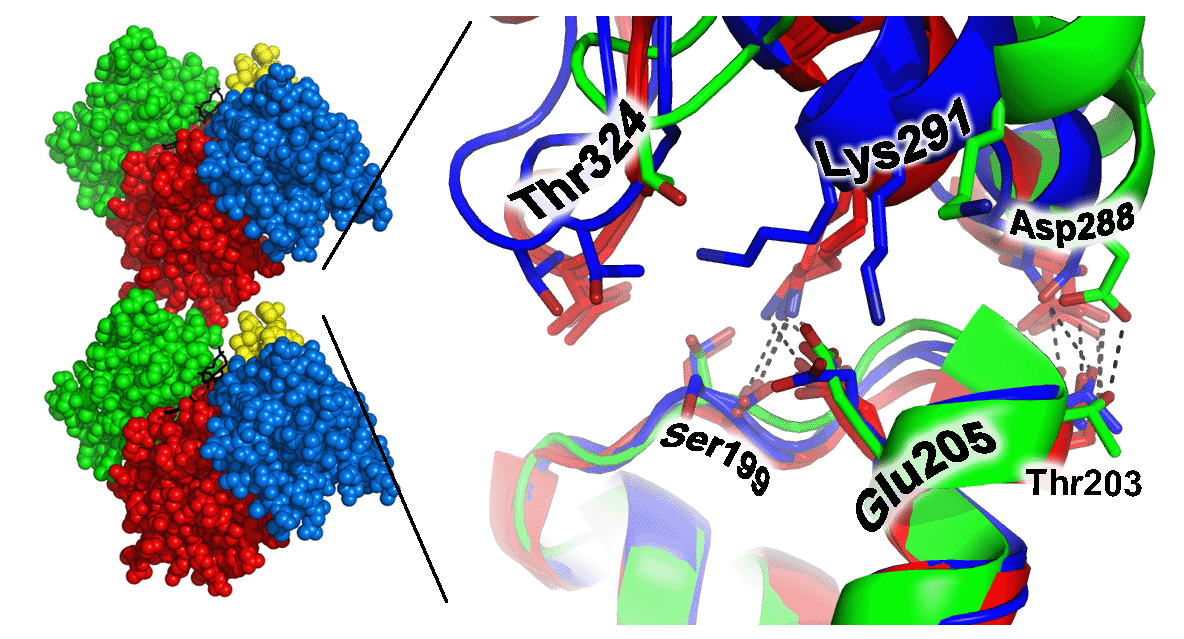
| Actin is one of the most abundant proteins in eukaryotic cells, where it plays key roles in cell shape, motility, and regulation. Actin exerts its functions by polymerization or self-assembly into a filamentous form, F-actin. The three-dimensional structure of actin is known in atomic detail only for the individual actin monomer. The structure of the F-actin filament is understood at considerably lower resolution, based upon pioneering fiber diffraction experiments at a resolution of about 9 Angstroms, performed by Ken Holmes in 1990, and based upon numerous subsequent electron microscopy studies at similar resolutions. Models at that resolution do not provide detailed information about the atomic interactions between individual protein subunits in the F-actin filament. Obtaining these structural details will be important in understanding the complex and dynamic behavior of actin at a mechanistic level. Filamentous structures present a notorious obstacle to obtaining atomic resolution information, since growth of crystals is generally prevented. One approach to this problem is to crystallize defined oligomeric species of the polymerizing protein. In collaboration with Emil Reisler's laboratory, we have determined multiple crystal structures of cross-linked actin dimers, thereby providing key evidence for specific atomic interactions between subdomain 3 and subdomain 4 from actin subunits in contact with each other along the longitudinal actin filament. |  |
Kudryashov, D.S., Sawaya, M.R., Adisetiyo, H.,
Norcross, T., Hegyi, G., Reisler,
E., Yeates, T.O. 2005.
The Crystal Structure of a Cross-linked Actin Dimer
Suggests a Detailed Molecular Interface in F-Actin. Proc Natl Acad Sci