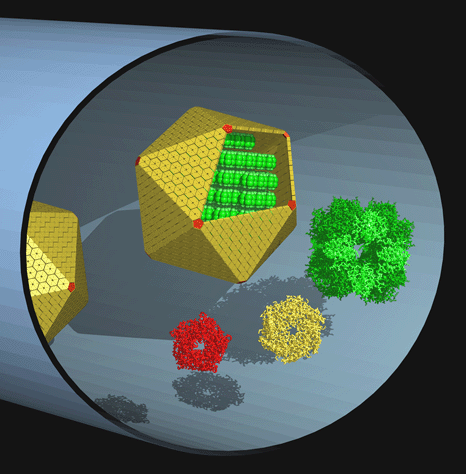
| The carboxysome is the best characterized example of a bacterial microcompartment. Bacterial microcompartments are simple organelles that act to sequester specific metabolic pathways inside many bacteria. The carboxysome encapsulates the enzymes required for CO2 fixation, namely RuBisCO and carbonic anhydrase, inside a proteinaceaous shell resembling a large viral capsid roughly 1200 Angstroms in diameter. Carboxysomes are present in all cyanobacteria and many chemoautotrophs, where they dramatically enhance the efficiency of carbon fixation by providing a concentrated source of CO2 for RuBisCO, whose relatively low reactivity and selectivity towards CO2 is problematic. Carboxysomes were first identified and characterized more than 30 years ago, but their structures have only recently come under systematic investigation. Cheryl Kerfeld, Shiho Tanaka, and Yingssu Tsai have determined the crystal structures of several proteins that make up the shell of the carboxysome. The hexameric units pack to form tight layers of molecules with small pores through which only small metabolites (e.g. bicarbonate, 3PGA, and 5RuBP) can pass. Pentameric proteins appear to form the vertices of the icosahedral shell. Three-dimensional structures of the component proteins make it possible now to ask specific questions about architecture, biochemical mechanisms, and evolutionary relationships. A number of intriguing questions remain, not the least of which is how the shell manages to recognize and encapsulate all the RuBisCO in the cell. Investigations of related but functionally distinct microcompartments, including those in E. coli and Salmonella, are just beginning. |  |
Tsai, Y., Sawaya,
M.R., Cannon, G.C., Cai, F.,
Williams, E.B., Heinhorst, S.,
Yeates, T.O., Tsai, Y., Tanaka, S., Sawaya, M.R.,