|
The cytosolic environment of most well-studied organisms is
chemically reducing. As a result, stabilizing disulfide
bonds are generally absent from cytosolic proteins, though
they are abundant in extracellular proteins, including those that are
secreted and those that reside in the bacterial periplasmic space.
However, this simplistic textbook view of protein disulfide bonding
is apparently violated by certain organisms. Based upon a crystal structure
of a hyperthermophilic protein he determined as a student in 2000, Eric Toth
predicted that certain thermophiles are
able to use disulfide bonding to stabilize their proteins against
extreme conditions. This claim was supported by Parag
Mallick in 2002 using computational approaches, and has since been validated by multiple
subsequent studies. The various studies included: a simple cysteine-counting exercise
(which showed that hyperthermophilic archaea show a clear abundance of
proteins having an even number of cysteine residues), genome-wide sequence-structure
mapping calculations (which showed a striking tendency of cysteine residues to be near other cysteine residues
in these organisms), and 2D oxidized-reduced SDS gels (which showed an abundance of proteins and protein-complexes
held together by disulfide bonds.
The widespread occurrence of disulfide bonds in these organisms helps explain the puzzle of how their proteins
are stabilized under such extreme conditions. It also paints a picture of protein disulfide bonding
and cellular redox state
that is more complex than previously anticipated. Our future efforts aim to exploit the expected
presence of disulfide bonds in proteins from these organisms to boost protein structure prediction algorithms.
References:
- Jorda J, Yeates TO. (2011).
Widespread disulfide bonding in proteins from thermophilic archaea.
Archaea. 2011. 2011:409156.
[Abstract]
Disulfide bonds are generally not used to stabilize proteins in the cytosolic compartments of bacteria or eukaryotic cells, owing to the chemically reducing nature of those environments. In contrast, certain thermophilic archaea use disulfide bonding as a major mechanism for protein stabilization. Here, we provide a current survey of completely sequenced genomes, applying computational methods to estimate the use of disulfide bonding across the Archaea. Microbes belonging to the Crenarchaeal branch, which are essentially all hyperthermophilic, are universally rich in disulfide bonding while lesser degrees of disulfide bonding are found among the thermophilic Euryarchaea, excluding those that are methanogenic. The results help clarify which parts of the archaeal lineage are likely to yield more examples and additional specific data on protein disulfide bonding, as increasing genomic sequencing efforts are brought to bear.
- Boutz DR, Cascio D, Whitelegge J, Perry LJ, Yeates TO. (2007).
Discovery of a thermophilic protein complex stabilized by topologically interlinked chains.
J. Mol. Biol.. May 2007. 368(5):1332-44.
[Abstract]
A growing number of organisms have been discovered inhabiting extreme environments, including temperatures in excess of 100 degrees C. How cellular proteins from such organisms retain their native folds under extreme conditions is still not fully understood. Recent computational and structural studies have identified disulfide bonding as an important mechanism for stabilizing intracellular proteins in certain thermophilic microbes. Here, we present the first proteomic analysis of intracellular disulfide bonding in the hyperthermophilic archaeon Pyrobaculum aerophilum. Our study reveals that the utilization of disulfide bonds extends beyond individual proteins to include many protein-protein complexes. We report the 1.6 A crystal structure of one such complex, a citrate synthase homodimer. The structure contains two intramolecular disulfide bonds, one per subunit, which result in the cyclization of each protein chain in such a way that the two chains are topologically interlinked, rendering them inseparable. This unusual feature emphasizes the variety and sophistication of the molecular mechanisms that can be achieved by evolution.
- Beeby M, O'Connor BD, Ryttersgaard C, Boutz DR, Perry LJ, Yeates TO. (2005).
The genomics of disulfide bonding and protein stabilization in thermophiles.
PLoS Biol.. Sep 2005. 3(9):e309.
[Abstract]
Thermophilic organisms flourish in varied high-temperature environmental niches that are deadly to other organisms. Recently, genomic evidence has implicated a critical role for disulfide bonds in the structural stabilization of intracellular proteins from certain of these organisms, contrary to the conventional view that structural disulfide bonds are exclusively extracellular. Here both computational and structural data are presented to explore the occurrence of disulfide bonds as a protein-stabilization method across many thermophilic prokaryotes. Based on computational studies, disulfide-bond richness is found to be widespread, with thermophiles containing the highest levels. Interestingly, only a distinct subset of thermophiles exhibit this property. A computational search for proteins matching this target phylogenetic profile singles out a specific protein, known as protein disulfide oxidoreductase, as a potential key player in thermophilic intracellular disulfide-bond formation. Finally, biochemical support in the form of a new crystal structure of a thermophilic protein with three disulfide bonds is presented together with a survey of known structures from the literature. Together, the results provide insight into biochemical specialization and the diversity of methods employed by organisms to stabilize their proteins in exotic environments. The findings also motivate continued efforts to sequence genomes from divergent organisms.
- Mallick P, Boutz DR, Eisenberg D, Yeates TO. (2002).
Genomic evidence that the intracellular proteins of archaeal microbes contain disulfide bonds.
Proc. Natl. Acad. Sci. U.S.A.. Jul 2002. 99(15):9679-84.
[Abstract]
Disulfide bonds have only rarely been found in intracellular proteins. That pattern is consistent with the chemically reducing environment inside the cells of well-studied organisms. However, recent experiments and new calculations based on genomic data of archaea provide striking contradictions to this pattern. Our results indicate that the intracellular proteins of certain hyperthermophilic archaea, especially the crenarchaea Pyrobaculum aerophilum and Aeropyrum pernix, are rich in disulfide bonds. This finding implicates disulfide bonding in stabilizing many thermostable proteins and points to novel chemical environments inside these microbes. These unexpected results illustrate the wealth of biochemical insights available from the growing reservoir of genomic data.
- Toth EA, Yeates TO. (2000).
The structure of adenylosuccinate lyase, an enzyme with dual activity in the de novo purine biosynthetic pathway.
Structure. Feb 2000. 8(2):163-74.
[Abstract]
Adenylosuccinate lyase is an enzyme that plays a critical role in both cellular replication and metabolism via its action in the de novo purine biosynthetic pathway. Adenylosuccinate lyase is the only enzyme in this pathway to catalyze two separate reactions, enabling it to participate in the addition of a nitrogen at two different positions in adenosine monophosphate. Both reactions catalyzed by adenylosuccinate lyase involve the beta-elimination of fumarate. Enzymes that catalyze this type of reaction belong to a superfamily, the members of which are homotetramers. Because adenylosuccinate lyase plays an integral part in maintaining proper cellular metabolism, mutations in the human enzyme can have severe clinical consequences, including mental retardation with autistic features.
|
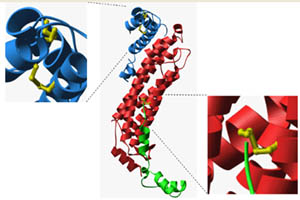
Initial structural evidence for disulfide bonding in P. aerophilum. (Adapted from Toth, et al.)
|
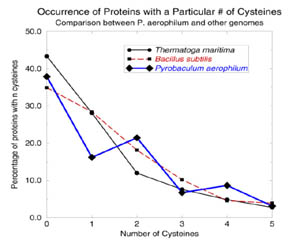
A simple cysteine-counting procedure detects disulfide bonding in hyperthermophiles. (Adapted from Mallick, et al.)
|
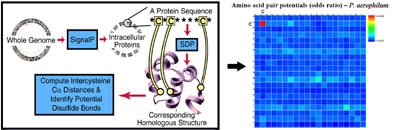
A genome-wide sequence-structure mapping approach shows clear evidence for widespread disulfide bonding in hyperthermophiles. The right panel highlights the tendency of cysteine residues to be mapped into proximity of other cysteine residues in
proteins from P. aerophilum. (Adapted from Mallick, et al. and Beeby, et al.)
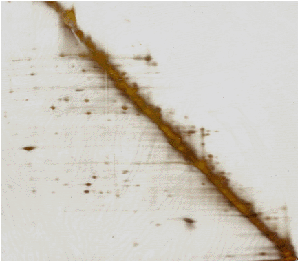
A 2D (oxidized-reduced) SDS gel of P. aerophilum lysate. (Adapted from Boutz, et al.)
|
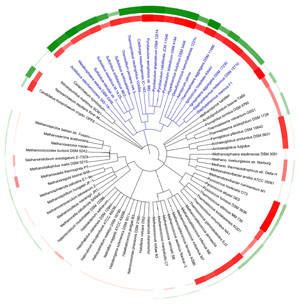
A current archaeal tree colored by optimal growth temperature (red) and predicted protein disulfide abundance (green) based on genomic calculations. The Crenarchaea are blue. (Adapted from Jorda, et al.)
|
|




