|
|
|
|

Other compounds are toxic. |
Safety:
Heavy atoms are toxic to humans, and uranyl compounds are radioactive.
When preparing heavy atom derivatives, special care must be taken to prevent
poisoning/irradiating yourself or others.
Handling precautions:
Wear gloves when handling heavy-atoms and avoid direct contact
with the skin. If contact occurs, wash the contaminated area
thoroughly with soap and water. Promptly clean up spills.
See the safety guide written by Dan Anderson for the use of heavy atoms. Here is a Standard Operating Procedure (SOP) for mercuric acetate, specific to the Eisenberg lab |
|
|
|
|
Selection:
Cystein, hisitidine, and methionine are reactive with
Class B heavy atoms (Hg, Au, Pt, Ir, etc.). Glutamic acid and Aspartic acid are
reactive toward Class A heavy atoms (Lanthanides, Actinides, e.g. U, Sm).
A concise review is given by Petsko in:
Petsko, G.A., "Perparation of Isomorphous Heavy-Atom Derivatives Methods in Enzymology, Volume 114, , pages 147-157. and more exhaustively in: Blundel & Johnson's book, "Protein Crystallography" pages 183-239. Recipes for iodide and cesium derivatives. Phasing with potassium iodide- a powerpoint presentation. Recipe for selenomethionyl derivatives. |
|
Selection:
Lanthanides and Actinides tend to have the strongest anomalous signal. To check how many anomalous electrons are in a given element see Ethan Merritt's X-ray Anomalous Scattering Tables. One should also consider how heavy atom reactivity varies with pH. Usually class B metal ions are more reactive at high pH. For more details see the useful pH range list at the Availability: There is a list of heavy atoms currently stocked in the Eisenberg Lab updated by Dan Anderson. |
|
The table below lists the most commonly cited heavy-atom derivatizing reagents as compiled from Macromolecular Structures for 1991-1994, based on Table 1 in: Rould, M.A. "Screening for Heavy-Atom Derivatives and Obtaining Accurate Isomorphous Differences" Methods in Enzymology, Volume 276, Part A pg.465) Perpared in part by Sylvie Doublie'. |
| Heavy-atom Reagent |
Highest stock concentration used |
Soak Time |
Citations |
| K2PtCl4 |
6mM |
10 days |
73 |
| KAu(CN)2 |
20mM |
- |
29 |
| Hg(CH3COO)2 |
50mM |
- |
29 |
| Pt(NH3)2Cl2 |
- |
- |
26 |
| HgCl2 |
20mM |
2 days |
25 |
| K3UO2F5 |
- |
- |
23 |
| Ethyl mercurithiosalicylate (Thimerosal) |
0.8mM |
10-30 days |
22 |
| (K/Na)AuCl4 |
- |
- |
22 |
| (Na/K)3IrCl6 |
5mM |
- |
21 |
| CH3CH2HgPO4 |
- |
- |
20 |
| K2PtCl6 |
5mM |
- |
19 |
| UO2(NO3)2 |
- |
- |
17 |
| K2Pt(NO2)4 |
10mM |
7 days |
17 |
| (CH3)3Pb(CH3COO) |
- |
- |
14 |
| CH3HgCl |
- |
- |
13 |
| p-Chloromercuribenzene sulfate (PCMBS) |
10mM |
- |
13 |
| K2Pt(CN)4 |
5mM |
- |
12 |
| Di-m-iodobis(ethylenediamine) diplatinum (PIP) |
- |
- |
12 |
| Pb(CH3COO)2 |
100mM |
1 day |
12 |
| K2HgI4 |
5mM |
days |
12 |
| Mersalyl |
0.9mM |
10-40 days |
12 |
| p-Chloromercuribenzoate (PCMB) |
0.8mM |
10-30 days |
11 |
| CH3Hg(CH3COO) |
- |
- |
11 |
| C(HgOOCH3)4 Tetrakis(mercuriacetoxy)methane (TAMM) |
- |
- |
10 |
| SmCl3 |
20mM |
- |
8 |
| K2OsO4 |
- |
- |
8 |
| (K/Na)2OsCl6 |
- |
- |
7 |
| 1,2-Diacetoxymercuri- 2,3-dimethoxybutane (Baker's dimercurial) |
10x Protein concentration |
- |
6 |
| 2-Chloromercuri-4-nitrophenol |
- |
- |
6 |
| AgNO3 |
- |
- |
5 |
| CH3CH2HgCl |
Saturated |
4 days |
5 |
| p-Hydroxymercuribenzoate |
- |
- |
5 |
|
|
|
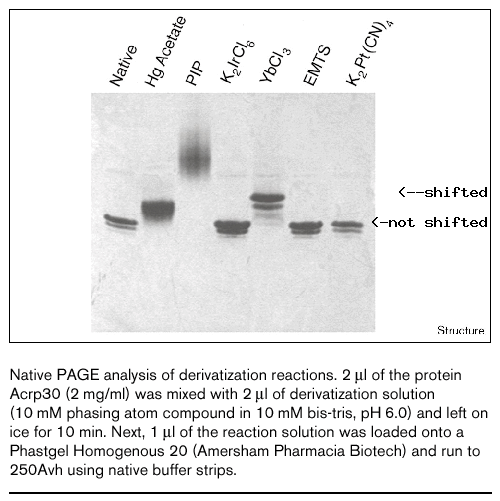
Screening:
Screening for heavy atom derivatives has never been easier!
Thanks to the observation by Bogon & Shapiro that the usefulness
of a heavy atom compound can be screened by means of a simple
native gel shift assay. Benefits of the method include the following
features:
See T. J. Boggon and L. Shapiro Structure 8, R143-R149, 2000. |
| Soaking:
Once you have identified the heavy atom conditions that produce
a native gel band shift, you can try soaking the heavy atom into your
crystal.
|