|
CHEM M230D Course Syllabus
|
|
Structural Molecular Biology Laboratory
Winter Quarter, 2026
|
Duilio Cascio
& Michael
R. Sawaya
Course Description: A laboratory course covering the
practical aspects of determining a protein crystal structure by X-ray crystallography.
Specifically, students will determine the structure of proteinase K, a 28
kDa enzyme, by performing all the steps of a typical crystallographic experiment:
crystallization, screening for heavy atom derivatives, collecting and reducing
data, space group determination, solving for the position of heavy atom sites,
calculating MIRAS phases, interpreting electron density, refining and validating
structure models, and making illustrative figures. Students will meet in
small groups, once a week by appointment for 1.5-2 hours. Five assignments
will be given to check understanding of course material and prepare the student
to publish a crystallographic journal article. Meeting Locations will vary
from week to week as noted below.
CHEM230A LECTURE MATERIALS available at https://people.mbi.ucla.edu/cascio/LECTURES/CHEM230A/.
When asked for a login name and password, please refer to the syllabus you
received on the first day of class.
Crystalliation and X-ray Lab Meeting Times 2026 Google Spreadsheet.
M230D LAB MATERIALS:
Experimental Procedures and Suggested Reading Materials:
 |
Crystallization and Heavy Atom Screening
January 5-9, 2026
Crystallization Facility, Boyer Hall Room 106
Suggested Reading/Viewing Materials:
1) Pre Crystallization Lab Video
2) What a crystallographer should know about a
protein before beginning crystallization trials by Michael Sawaya.
3) Heavy Atom Safety, Selection, and Screening
by Michael Sawaya (2000).
4) Screening for Phasing Atoms in Protein Crystallography
by Boggon & Shapiro (2000).
Experiments Performed in Lab:
1) Crystallization of Proteinase K and complex with
PMSF
2) Heavy Atom Derivative Screening by Native Gel
Electrophoresis
3) Preparation of Heavy Atom derivatives
Heavy Atom Soaking
January 8-16, 2026
X-ray Lab, Boyer Hall Room 124
|
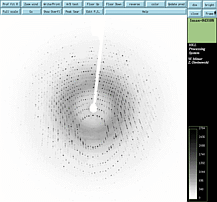 |
Cryo-crystallography
& Data Collection
January 12-16, 2026
X-ray Lab, Boyer Hall Room 124
Suggested Reading Materials:
1) Data Collection Strategies by Zbigniew
Dauter (1999).
2) Solvent Content of Protein Crystals by
B.W. Matthews (1968).
Activities In Lab:
1) Cryo-crystallographic technique video (WMV file 65 Mb, open with Windows Media Player)
2) Practice removing and storing crystals for synchrotron
trip
3) Initiate data collection
Data
Reduction
January 19-23, 2026
in Grad Student Computer Lab, Young Hall Room 4067
Activities In Lab:
1) Data Reduction
Assignments: "Table 1" and crystal data collection reports
completed in lab |
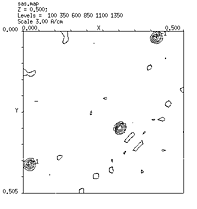 |
Difference
Patterson Maps & Determination of Heavy Atom Sites
January 26-30, 2026
in Grad Student Computer Lab, Young Hall Room 4067
Suggested Reading Materials:
1) Chapter 6 (page 101-127) from Crystallography
Made Crystal Clear by Gale Rhodes.
Activities in Lab:
1) Familiarization with simple Unix commands.
2) Calculation of isomorphous and anomalous difference
Patterson maps.
Assignments: Patterson reports completed in lab
|
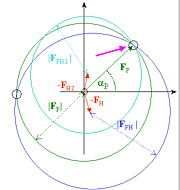 |
Phasing
& Electron Density Map Calculation
January 26-30, 2026
in Computer Lab, Young Hall Room 4067
Suggested Reading Materials:
1) Chapter 6 (page 101-127) from Crystallography
Made Crystal Clear by Gale Rhodes.
Activities in Lab:
1) Phasing with Shelx and HKL2MAP
2) Solvent Flattening
3) Chose correct handedness
4) Calculate electron density
5) View density with "COOT"
Assignments: Phasing reports completed in lab
|
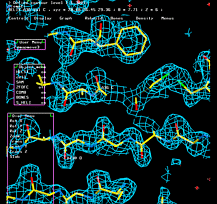 |
Model
Building part I
Feb 2-13, 2026
in Computer Lab, Young Hall Room 4067
Suggested Reading Materials:
1) Electron density map intepretation by T. A. Jones
and M. Kjeldgaard.
Activities in Lab:
1) Placing Alpha helices and beta strands in density.
2) Assigning protein sequence to density
3) Run & evaluate automatic chain tracing program
Assignments: Complete building an atomic model of Proteinase K by March 2-6, 2026. Sooner is better.
|
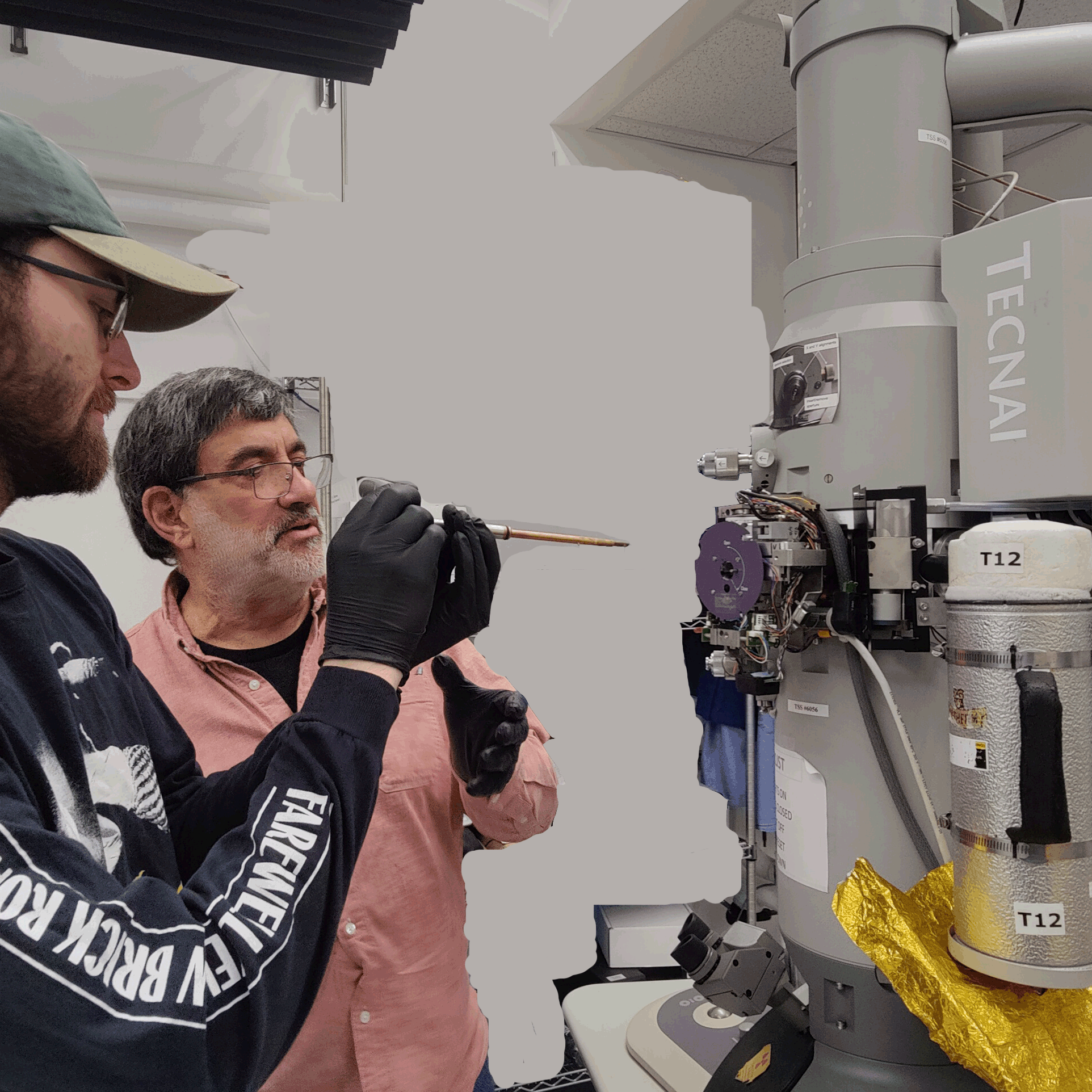 |
EM demo, sample preparation, T12 training
Feb 16-20, 2026
in Boyer Hall Room 105
Suggested Reading Materials:
1) T12 manual
2) Sample Preparation protocol
Activities in Lab:
1) Preparation of biological samples for negative stain TEM.
2) Basic operating procedures for loading a viewing a sample on a T12 electron microscope
|
 |
CryoSPARC demonstration
Feb 23-27, 2026
in Computer Lab, Young Hall Room 4067
Suggested Reading Materials:
1) CryoSPARC tutorial
Activities in Lab:
1) Motion Correction of CryoEM images
2) Contrast Transfer function (CTF) Estimation
3) Particle Picking and Extraction
4) 2D classification
5) Ab initio 3D reconstruction
6) Homogeneous refinement
|
 |
Model Building part II, Refinement, and Structure Validation
Mar 2-6, 2026
in Computer Lab, Young Hall Room 4067
Suggested Reading Materials:
1) None yet.
Activities in Lab:
1) Calculation of difference Fourier electron density
maps.
2) Evaluation of maps
3) Refinement of Structure
4) Run Procheck, Verify3D and Errat
Assignments: Final structure reports completed in lab
|
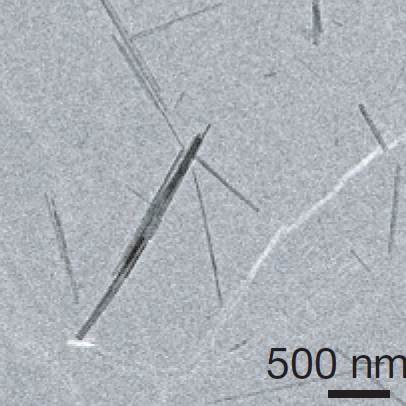 |
MicroED on Talos Electron Microscope
March 9-13, 2026
in Young Hall Room 1290
Suggested Reading Materials:
1) Talos tutorial
Activities in Lab:
1) Load microcrystals in Talos microscope
2) View and select a microcrystal of interest
3) Collect diffraction data as a tilt series of ~70 degrees
4) Process diffaction data with XDS
5) Solve phase problem using direct methods
6) Observe atomic structure
|
Pymol tutorial
[Overview] · [Facilities] · [People] · [Services] · [Lectures] · [BioLinks] ·
[Stats] · [Search]
|








